| 3 Signal Transduction, Membrane Receptors, Second Messengers, and Regulation of Gene Expression
|
| The human body is composed of billions of cells, each with a distinct function. However, the function of cells is tightly coordinated and integrated by external chemical signals, including hormones, neurotransmitters, growth factors, odorants, and products of cellular metabolism that serve as chemical messengers and provide cell-to-cell communication. Light and mechanical and thermal stimuli are physical external signals that also coordinate cellular function. Chemical and physical messengers interact with receptors located in the plasma membrane, cytoplasm, and nucleus. Interaction of these messengers with receptors initiates a cascade of signaling events that mediate the response to each stimulus. These signaling pathways ensure that the cellular response to external messengers is specific, amplified, tightly regulated, and coordinated. This chapter provides an overview of how cells communicate via external messengers and discusses the receptors and intracellular signaling pathways that process external information into a highly coordinated cellular response. In subsequent chapters, details on signaling pathways in the nervous system, muscle, cardiovascular system, respiratory system, gastrointestinal system, kidneys, and endocrine system will be discussed in greater detail.
|
| CELL-TO-CELL COMMUNICATION
|
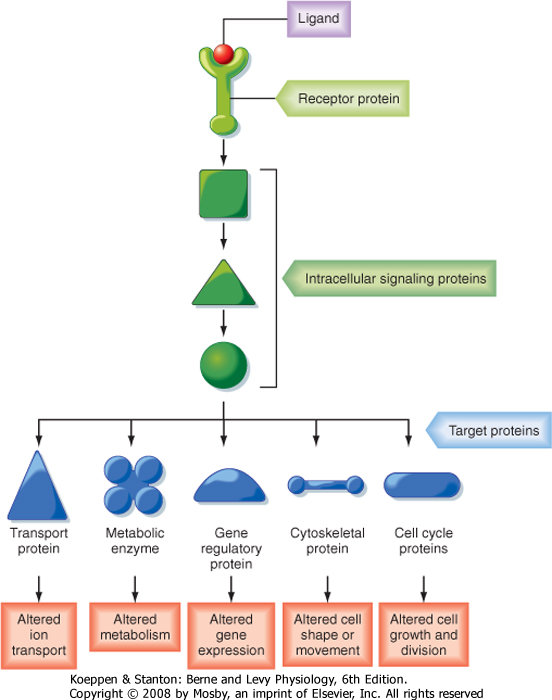
| An overview of how cells communicate with each other is presented in Figure 3-1. Cells communicate by releasing extracellular signaling molecules (e.g., hormones and neurotransmitters) that bind to receptor proteins located in the plasma membrane, cytoplasm, or nucleus. This signal is transduced into the activation, or inactivation, of one or more intercellular messengers by interacting with receptors. Receptors interact with a variety of intracellular signaling proteins, including kinases, phosphatases, and GTP-binding proteins (G proteins). These signaling proteins interact with and regulate the activity of target proteins and thereby modulate cellular function. Target proteins include, but are not limited to, ion channels and other transport proteins, metabolic enzymes, cytoskeletal proteins, gene regulatory proteins, and cell cycle proteins that regulate cell growth and division. Signaling pathways are characterized by (1) multiple, hierarchical steps; (2) amplification of the hormone-receptor binding event, which magnifies the response; (3) activation of multiple pathways and regulation of multiple cellular functions; and (4) antagonism by constitutive and regulated feedback mechanisms, which minimize the response and provide tight regulatory control over these signaling pathways. A brief description of how cells communicate follows. Readers who desire a more in-depth presentation of this material are encouraged to consult one of the many cellular and molecular biology textbooks currently available.
|
| Cells in higher animals release hundreds of signaling molecules, including peptides and proteins (e.g., insulin), catecholamines (e.g., epinephrine and norepinephrine), steroid hormones (e.g., aldosterone, estrogen), iodothyronines (e.g., thyroid hormones, including thyroxine [T4] and triiodothyronine [T3]), eicosanoids (e.g., prostaglandins, leukotrienes, thromboxanes, and prostacyclins), and other small molecules, including amino acids, nucleotides, ions (e.g., Ca++), and gases, such as nitric oxide (NO) and carbon dioxide (CO2), into the extracellular space by the processes of exocytosis and diffusion. Secretion of signaling molecules is cell type specific. For example, beta cells in the pancreas release insulin, which regulates glucose uptake into cells. The ability of a cell to respond to a specific signaling molecule depends on the expression of receptors that bind the signaling molecule with high affinity and specificity. Receptors are located in the plasma membrane, the cytosol, and the nucleus (Fig. 3-2).
|
| page 34 |  | | page 35 |
| Figure 3-1 An overview of how cells communicate. A ligand (i.e., hormone or neuro-transmitter) binds to a receptor, which may be in the plasma membrane, cytosol, or nucleus. Binding of ligand to a receptor activates intracellular signaling proteins, which interact with and regulate the activity of one or more target proteins to change cellular function. Signaling molecules regulate cell growth, division, and differentiation and influence cellular metabolism. In addition, they modulate the intracellular ionic composition by regulating the activity of ion channels and transport proteins. Signaling molecules also control cytoskeletal-associated events, including cell shape, division, and migration and cell-to-cell and cell-to-matrix adhesion. (Redrawn from Alberts B et al: Molecular Biology of the Cell, 4th ed. New York, Garland Science, 2002.) |

|
| Figure 3-2 Signaling molecules, especially ones that are hydrophilic and cannot cross the plasma membrane, directly bind to their cognate receptors located in the plasma membrane. Other signaling molecules, including steroid hormones, triiodothyronines, retinoic acids, and vitamin D, bind to carrier proteins in blood and readily diffuse across the plasma membrane, where they bind to cognate so-called nuclear receptors in the cytosol or nucleus. Both classes of receptors, when ligand bound, regulate gene transcription. (Redrawn from Alberts B et al: Molecular Biology of the Cell, 4th ed. New York, Garland Science, 2002.) |
| page 35 |  | | page 36 |
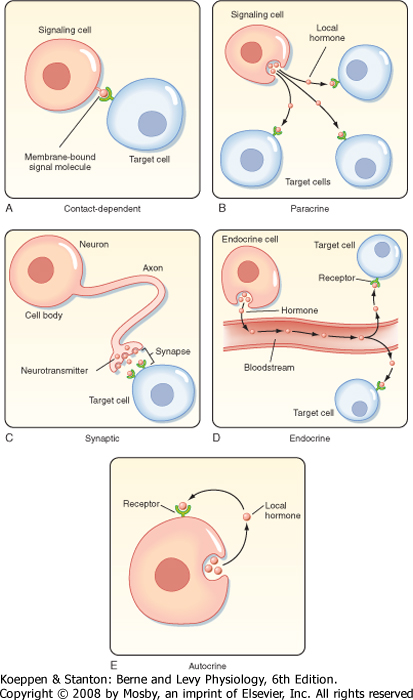
| Figure 3-3 Cell-to-cell communication is mediated by five basic mechanisms, described in the text. (Redrawn from Alberts B et al: Molecular Biology of the Cell, 4th ed. New York, Garland Science, 2002.) |
| page 36 |  | | page 37 |
|
Table 3-1.
Classes of Membrane Receptors |
| Receptor Class | Ligand | Signal Transduction Pathway |
| | Extracellular ligand: | Membrane currents: |
| | GABA | Cl- |
| | ACh | Na+, K+, Ca++ |
| | ATP | Ca++, Na+ |
| 1. Ion channel | | K+ |
| | Intracellular ligand: | Na+, K+ |
| | cAMP | Na+, K+ |
| | cGMP | Ca++ |
| | InsP3 | Ca++ |
| | Ca++ | |
| | Neurotransmitters | βγ Subunits activate ion channels |
| | | α Subunit activates enzymes: |
| 2. G protein | Peptides
Odorants
Cytokines lipids | Cyclases, which generate cAMP and cGMP; phospholipases, which generate InsP3 and diacylglycerol; and phospholipases, which generate arachidonic acid and its metabolites |
| | | Monomeric G proteins |
| 3. Catalytic | ANP
Insulin, EGF | Receptor guanylyl cyclase
Receptor tyrosine kinase |
| | Steroid hormones: | Bind to regulatory sequences in DNA and increase or decrease gene transcription |
| | Mineralocorticoids | |
| | Glucocorticoids | |
| | Androgens | |
| | Estrogens | |
| | Progestins | |
| 4. Nuclear | Miscellaneous hormones: | Bind to regulatory sequences in DNA and increase or decrease gene transcription |
| | Thyroid | |
| | Vitamin D | |
| | Retinoic acid | |
| | Prostaglandins | |
|
ACh, acetylcholine; ANP, atrial natriuretic peptide; cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanosine monophosphate; EGF, epidermal growth factor; GABA, γ-aminobutyric acid; InsP3, inositol 1,4,5-triphosphate; PDGF, platelet-derived growth factor.
|
| Signaling molecules can act over long or short distances and can require cell-to-cell contact or very close cellular proximity (Fig. 3-3). Contact-dependent signaling is important during development and in immune responses. Molecules that are released and act locally are called paracrine or autocrine hormones. Paracrine signals are released by one type of cell and act on another type; they are usually taken up by target cells or rapidly degraded (within minutes) by enzymes. Autocrine signaling involves the release of a molecule that affects the same cell or other cells
of the same type. Synaptic signaling occurs when neurons transmit electrical signals along their axons and release neurotransmitters at synapses that affect the function of other neurons or cells that are distant from the neuron cell body. The physical relationship between the nerve terminal and the target cells ensures that the neurotransmitter is delivered to a specific cell. Details on synaptic signaling are discussed in Chapter 6. Endocrine signals are hormones that are secreted into the blood and are widely dispersed in
the body. Details on endocrine signaling are discussed in Chapter 37.
|
| In addition to paracrine, autocrine, endocrine, and synaptic signaling, cell-to-cell communication also occurs via the gap junctions that form between adjacent cells (see Chapter 1). Gap junctions are specialized junctions that allow intracellular signaling molecules, generally less than 1200 Da in size, to diffuse from the cytoplasm of one cell to an adjacent cell. The permeability of gap junctions is regulated by cytosolic [Ca++], [H+], and cAMP and by the membrane potential. Gap junctions also allow cells to be electrically coupled, which is vitally important for the coordinated activity of cardiac and smooth muscle cells (see Chapters 13 and 14).
|
| The speed of a response to an extracellular signal depends on the mechanism of delivery. Endocrine signals are relatively slow (seconds to minutes) because time is required for diffusion and blood flow to the target cell, whereas synaptic signaling is extremely fast (milliseconds). If the response involves changes in the activity of proteins in the cell, the response may occur in milliseconds to seconds. However, if the response involves changes in gene expression and the de novo synthesis of proteins, the response may take hours to occur, with days required to achieve a maximal response. For example, the stimulatory effect of aldosterone on sodium transport by the kidney requires days to fully develop (see Chapter 34).
|
| The response to a particular signaling molecule also depends on the ability of the molecule to reach a particular cell, on expression of the cognate receptor (i.e., receptors that recognize a particular signaling molecule or ligand with a high degree of specificity), and on the cytoplasmic signaling molecules that interact with the receptor. Thus, signaling molecules frequently have many different effects that are dependent on the cell type. For example, the neurotransmitter acetylcholine stimulates contraction of skeletal muscle but decreases the force of contraction in heart muscle. This is due to the fact that skeletal muscle and heart cells express different acetylcholine receptors.*
|
| All signaling molecules bind to specific receptors that act as signal transducers, thereby converting a ligand-receptor binding event into intracellular signals that affect cellular function. Receptors can be divided into two basic classes based on their structure and mechanism of action: membrane receptors and nuclear receptors (Table 3-1).
|
| page 37 |  | | page 38 |
| Plasma Membrane Receptors
|
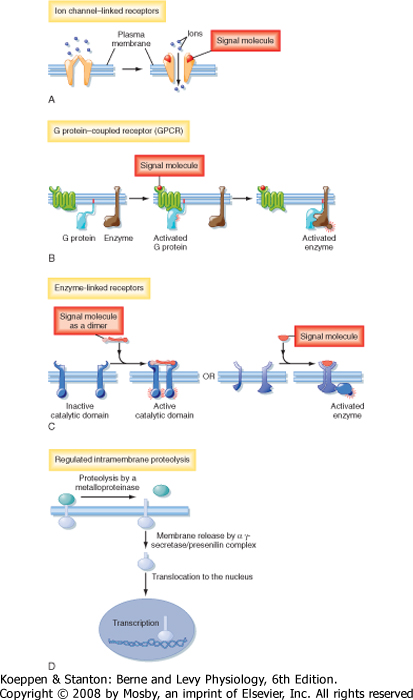
| There are four major types of plasma membrane receptors defined by the intracellular signaling pathways that they use: ion channel-linked receptors, G protein-coupled receptors (GPCRs), catalytic receptors, and a fourth class of transmembrane receptors that when activated, release transcription factors that undergo proteolytic cleavage and liberate a cytosolic fragment that enters the nucleus and modulates gene expression (Fig. 3-4).
|
| Ion channel-linked receptors, also known as ligand-gated ion channels, mediate direct and rapid synaptic signaling between electrically excitable cells (Fig. 3-4, A). Neurotransmitters bind to the receptors and either open or close the ion channel, thereby changing the ionic permeability of the plasma membrane and altering the membrane potential. For examples and more details, see Chapter 2.
|
| GPCRs regulate the activity of other proteins, such as enzymes and ion channels (Fig. 3-4, B). In this type of receptor the interaction between the receptor and the target protein is mediated by heterotrimeric G proteins, which are composed of α, β, and γ subunits. Stimulation of G proteins by ligand-bound receptors activates or inhibits downstream target proteins that regulate signaling pathways if the target protein is an enzyme or change membrane ion permeability if the target protein is an ion channel.
|
| Catalytic receptors either function as enzymes or are associated with and regulate enzymes (Fig. 3-4, C). Most enzyme-linked receptors are protein kinases or are associated with protein kinases, and ligand binding causes the kinases to phosphorylate a specific subset of proteins on specific amino acids, which in turn activates or inhibits protein activity.
|
| Some membrane proteins do not fit the classic definition of receptors, yet they subserve a receptor-like function in that they recognize extracellular signals and transduce the signals into an intracellular second messenger that has a biological effect. For example, on activation by a ligand, some membrane proteins undergo regulated intramembrane proteolysis (RIP), which elaborates a cytosolic peptide fragment that enters the nucleus and regulates gene expression (Fig. 3-4, D). In this signaling pathway, binding of ligand to a plasma membrane receptor leads to ectodomain shedding, facilitated by members of the metalloproteinase-disintegrin family, and produces a carboxy-terminal fragment that is the substrate for γ-secretase. γ-Secretase induces RIP, thereby releasing an intracellular domain of the protein that enters the nucleus and regulates transcription (Fig. 3-4, D). The most well characterized example of RIP is the sterol regulatory element-binding protein (SREB), a transmembrane protein expressed in the membrane of the endoplasmic reticulum. When cellular cholesterol levels are low, SREB undergoes RIP and the proteolytically cleaved fragment is translocated into the nucleus, where it transcriptionally activates genes that promote cholesterol biosynthesis.
|
| Alzheimer's disease (AD) is a progressive neurodegenerative brain disease characterized by the formation of amyloid plaques. In AD, regulated intramembrane proteolysis of amyloid β-protein precursor (APP) causes the accumulation of amyloid β-protein (Aβ), which forms amyloid plaques that contribute to the pathogenesis of AD. APP is a type I transmembrane protein (i.e., its spans the membrane only once). After ectodomain shedding, its sequential proteolysis by β-secretase and γ-secretase produces the Aβ40 and Aβ42 peptides that are normally produced throughout life but accumulate in individuals with Alzheimer's disease. Missense mutations in presenilins (PS1 and PS2), proteins that regulate γ-secretase protease activity, enhance the production of Aβ42, which is more hydrophobic and prone to aggregation into amyloid fibrils than the more abundant Aβ40 protein is. |
 |
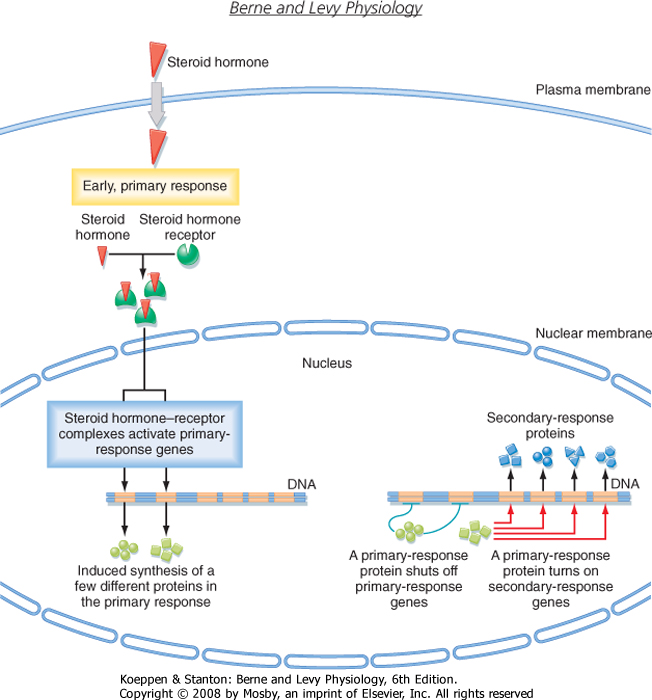
| Several classes of small hydrophobic molecules, including steroid hormones, thyroid hormones, retinoids, and vitamin D, are bound to plasma proteins, have a long biological half-life (hours to days), diffuse across the plasma membrane, and bind to nuclear receptors (Fig. 3-5). Some nuclear receptors, such as those that bind cortisol and aldosterone, are located in the cytosol and enter the nucleus after binding to hormone, whereas other receptors, including the thyroid hormone receptor, are bound to DNA in the nucleus, even in the absence of hormone. In both cases, inactive receptors are bound to inhibitory proteins, and binding of hormone results in dissociation of the inhibitory complex. Hormone binding causes the receptor to bind coactivator proteins that activate gene transcription. Once activated, the hormone-receptor complex binds to DNA and regulates the transcription of specific genes. The thyroid hormone-receptor complex binds to DNA complexes adjacent to the genes that the hormone regulates. Activation of specific genes usually occurs in two steps: an early primary response (≈30 minutes), which activates genes that stimulate other genes to produce a delayed (hours to days) secondary response (Fig. 3-5). Each hormone elicits a specific response based on cellular expression of the cognate receptor, as well as cell type-specific expression of gene regulatory proteins that interact with the activated receptor to regulate the transcription of a specific set of genes (see Chapter 37 for more details). In addition to steroid receptors that regulate gene expression, recent evidence suggests that there are also membrane and juxtamembrane steroid receptors that mediate the rapid, nongenomic effects of steroid hormones.
|
| page 38 |  | | page 39 |
| Figure 3-4 Classes of plasma membrane receptors. See text for details. (Redrawn from Alberts B et al: Molecular Biology of the Cell, 4th ed. New York, Garland Science, 2002.) |
| page 39 |  | | page 40 |
| Figure 3-5 Steroid hormones stimulate the transcription of early-response genes and late-response genes. See text for details. (Redrawn from Alberts B et al: Molecular Biology of the Cell, 4th ed. New York, Garland Science, 2002.) |
| RECEPTORS AND SIGNAL TRANSDUCTION PATHWAYS
|
| Hormones bind to receptors and the signal is translated to effector proteins inside the cell by intracellular signaling proteins. Plasma membrane receptors relay signals via intracellular signaling pathways. Nuclear receptors relay signals primarily through regulation of gene expression. Receptors amplify and integrate signals, as well as down-regulate and desensitize signals, which reduces or terminates the response, even in the presence of hormone.
|
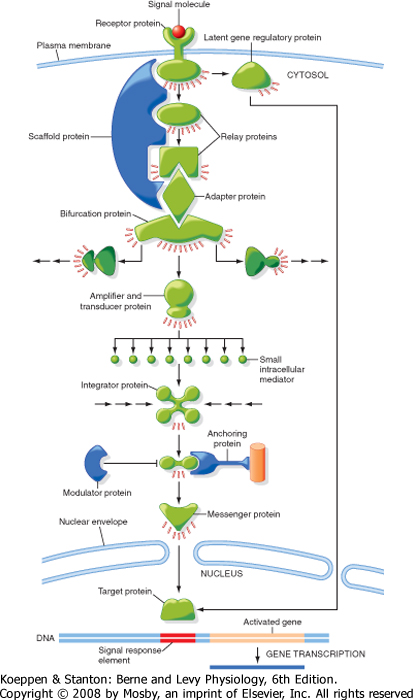
| Intracellular signaling molecules-so-called second messengers (the first messenger of the signal is the ligand that binds to the receptor)-include small molecules such as cAMP, cGMP, Ca++, and diacylglycerol (DAG). Signaling pathways often include dozens of small molecules that form complicated networks within the cell (Fig. 3-6). Some proteins in the intracellular signaling pathways relay the signal by passing the message from one protein to another. Other proteins carry the signal from one region of the cell to another, for example, from the cytosol to the nucleus. Many proteins, usually enzymes or ion channels, amplify the signal either by producing large amounts of additional signaling molecules or by activating a large number of downstream signaling proteins. Transducer proteins convert the signal into a different form. The enzyme that makes cAMP, adenylyl cyclase, transduces a signal (activation of a G protein) and amplifies the signal by generating large amounts of cAMP. Other types of signaling proteins include those that integrate multiple signals.
|
| Intracellular signals also act as molecular switches: when a signal is received, they switch from an inactive to an active form or vice versa, until another signaling molecule switches them off. Signaling complexes, composed of multiple proteins that interact physically, enhance the speed, efficiency, and specificity of signaling. Cells can also adjust rapidly to signaling molecules. Cells can respond quickly and in a graded manner to increasing concentrations of hormone, and the effect of a signaling molecular can either be long- or short-lived.
|
| page 40 |  | | page 41 |
| Figure 3-6 Overview of how intracellular signals are amplified and integrated. Signaling pathways often include dozens of small molecules that form complicated networks within the cell. Some signaling proteins relay the signal by passing the message from one protein to another. Other proteins carry the signal from one region of the cell to another. Many proteins amplify the signal either by producing large amounts of additional signaling molecules or by activating a large number of downstream signaling proteins. See text for more details. (Redrawn from Alberts B et al: Molecular Biology of the Cell, 4th ed. New York, Garland Science, 2002.) |
| page 41 |  | | page 42 |
| Cells can adjust their sensitivity to a signal by adaptation or desensitization, whereby prolonged exposure to a hormone decreases the cell's response over
time. Adaptation allows cells to respond to changes in hormone levels rather than to absolute levels. Adaptation is a reversible process that can involve a reduction in the number of receptors expressed in the plasma membrane, inactivation of receptors, and changes in signaling proteins mediating the downstream effect of the receptors.
|
| Table 3-1 summarizes the four general classes of receptors and provides a few examples of the signal transduction pathways associated with each class of receptors.
|
| Ion Channel-Linked Signal Transduction Pathways
|
| This class of receptors transduces a chemical signal into an electrical signal, which elicits a response. For example, activation of the ryanodine receptor (RyR), located in the membrane of the sarcoplasmic reticulum of skeletal muscle, by Ca++, caffeine, ATP, or metabolites of arachidonic acid releases Ca++ into the cytosol, which facilitates muscle contraction (see Chapter 12 for details).
|
| G Protein-Coupled Signal Transduction Pathways
|
| G proteins couple to more than 1000 different receptors and thereby mediate the cellular response to an incredibly diverse set of signaling molecules, including hormones, neurotransmitters, peptides, and odorants. G proteins are heterotrimeric complexes composed of three subunits, α, β, and γ. There are 16 α subunits, 5 β subunits and 11 γ subunits. These α, β, and γ subunits can assemble into hundreds of different combinations and thereby interact with a diverse number of receptors and effectors. The assembly of subunits and the association with receptors and effectors depend on the cell type.
|
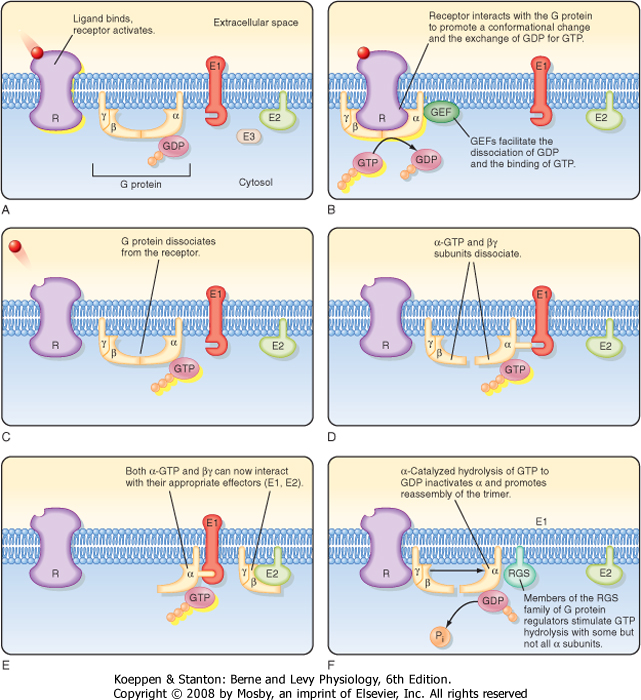
| An overview of G protein activation and inactivation is illustrated in Figure 3-7. In the absence of ligand, G proteins are inactive and form a heterotrimeric complex in which GDP binds to the α subunit. When a ligand binds to a receptor, the activated receptor interacts with the α, β, γ complex and induces a conformational change that promotes the release of GDP and binding of GTP to the α subunit. Binding of GTP to the α subunit stimulates dissociation of the α subunit from the heterotrimeric complex and results in release of the α subunit from the βγ dimer, each of which can interact with and regulate downstream effectors such as adenylyl cyclase and phospholipases. G proteins are activated by guanine nucleotide exchange factors (GEFs), which facilitate the dissociation of GDP and binding of GTP, and are inactivated by GTPase-accelerating proteins (GAPS), which enhance G protein GTPase activity. Activation of downstream effectors by the α subunit and βγ dimer is terminated when the α subunit hydrolyzes the bound GTP to GDP and Pi. The α subunit bound to GDP reassociates with the βγ dimer and terminates the activation of effectors. Hydrolysis of GTP by the α subunit is facilitated by a family of proteins known as RGS proteins (regulation of G protein signaling), which facilitate inactivation of signaling.
|
| Another way to terminate signaling through a GPCR involves desensitization and endocytic removal of receptors from the plasma membrane. Binding of hormone to a GPCR increases the ability of GPCR kinases (GRKs) to phosphorylate the intracellular domain of GPCRs, which recruits proteins called β-arrestins to bind to the GPCR. β-Arrestins inactivate the receptor and promote endocytic removal of the GPCR from the plasma membrane. GRK/β-arrestin inactivation with endocytosis of GPCRs is an important mechanism whereby cells down-regulate (desensitize) a response during prolonged exposure to elevated hormone levels.
|
| Activated α subunits couple to a variety of effector proteins, including adenylyl cyclase, phosphodiesterases, and phospholipases (A2, C, and D). A very common downstream effector of G proteins is adenylyl cyclase, which facilitates the conversion of ATP to cAMP (Fig. 3-8, A). When a ligand binds to a receptor that interacts with a G protein composed of an α subunit of the αs class, adenylyl cyclase is activated, thereby increasing cAMP levels and as a result activating protein kinase A (PKA). By phosphorylating specific serine and threonine residues on proteins, PKA regulates effector protein activity. In contrast, when a ligand binds to a receptor that interacts with a G protein composed of an α subunit of the αi class, adenylyl cyclase is inhibited, thereby reducing cAMP levels and consequently reducing PKA levels. cAMP also regulates some effector proteins directly, such as ion-gated channels. cAMP is degraded to AMP by cAMP phosphodiesterases, which are inhibited by caffeine and other methylxanthines. Thus, caffeine can prolong a cellular response mediated by cAMP and PKA. In addition to signaling in the cytoplasm, the catalytic subunit of PKA can enter the nucleus of cells and phosphorylate and activate the transcription factor cAMP response element-binding (CREB) protein. Phospho-CREB protein increases the transcription of many genes. Hence cAMP has many cellular effects, including direct and indirect effects mediated by PKA.
|
| G proteins also regulate phototransduction (Fig. 3-8, B). In rod cells in the eye, absorption of light by rhodopsin activates the G protein transducin, which via the αt subunit activates cGMP phosphodiesterase. Activation of this phosphodiesterase lowers the concentration of cGMP and thereby closes a cGMP-activated cation channel. The ensuing change in cation channel activity alters the membrane voltage. The exquisite sensitivity of rods to light-rods can detect a single photon of light-is due to the abundance of rhodopsin in rods and amplification of the signal (a photon of light) by the G protein-cGMP phosphodiesterase-cGMP channel signaling pathway (see Chapter 8 for more details).
|
| page 42 |  | | page 43 |
| Figure 3-7 Cycle of heterotrimeric G protein activation and inactivation. The same cycle is involved in the activation and inactivation of small, monomeric G proteins. (Redrawn from Boron W, Boulpaep E: Medical Physiology. Philadelphia, Saunders, 2003.) |
| G proteins also regulate phospholipases, a family of enzymes that modulate a variety of signaling pathways (Fig. 3-8, C). Ligands that activate receptors that are coupled to the αq subunit stimulate phospholipase C,
an enzyme that converts phosphatidylinositol 4,5-biphosphate (PIP2) to 1,4,5-inositol triphosphate (InsP3) and DAG (Fig. 3-8, C). InsP3 is a second messenger that diffuses to the endoplasmic reticulum, where it activates a ligand-activated Ca++ channel to release Ca++ into the cytosol, whereas DAG activates protein kinase C (PKC), which phosphorylates effector proteins. As noted earlier, both Ca++ and PKC influence effector proteins, as well as other signaling pathways, to elicit responses.
|
| page 43 |  | | page 44 |
| Figure 3-8 Heterotrimeric G proteins regulate (A) adenylyl cyclase and thereby modulate cAMP and PKA levels; (B) phosphodiesterases, which modulate cGMP and cAMP levels; and (C) phospholipases, which release DAG. In turn, DAG activates PKC and InsP3, which stimulate Ca++ release from the endoplasmic reticulum. (Redrawn from Boron W, Boulpaep E: Medical Physiology. Philadelphia, Saunders, 2003.) |
| Ligand binding to GPCRs can also activate phospholipase A2, an enzyme that releases arachidonic acid from membrane phospholipids (Fig. 3-9). Arachidonic acid can be released from cells and thereby regulates neighboring cells or stimulates inflammation. It can also be retained within cells, where it is incorporated
into the plasma membrane or is metabolized in the cytosol to form intracellular second messengers that affect the activity of enzymes and ion channels (Fig. 3-9). In one pathway, cytosolic cyclooxygenases facilitate the metabolism of arachidonic acid to prostaglandins, thromboxanes, and prostacyclins. Prostaglandins mediate aggregation of platelets, cause constriction of the airways, and induce inflammation. Thromboxanes also induce platelet aggregation and constrict blood vessels, whereas prostacyclin inhibits platelet aggregation and dilates blood vessels. In a second pathway of arachidonic acid metabolism, the enzyme 5-lipoxygenase initiates the conversion of arachidonic acid to leukotrienes, which participate in allergic and inflammatory responses, including those causing asthma, rheumatoid arthritis, and inflammatory bowel disease. The third pathway of arachidonic acid metabolism is initiated by epoxygenase, an enzyme that facilitates the generation of hydroxyeicosatetraenoic acid (HETE) and cis-epoxyeicosatrienoic acid (EET). HETE and EET increase release of Ca++ from the endoplasmic reticulum and stimulate cell proliferation.
|
| Ca++ is also an intracellular messenger that elicits cellular effects via Ca++-binding proteins, most notably calmodulin (CaM). When Ca++ binds to CaM, its conformation is altered and the structural change in CaM allows it to bind to and regulate other signaling proteins, including cAMP phosphodiesterase, an enzyme that degrades cAMP to AMP, which is inactive and unable to activate PKA. By binding to CaM-dependent kinases, CaM also phosphorylates specific serine and threonine residues in many proteins, including myosin light-chain kinase, which facilitates smooth muscle contraction (see Chapter 14).
|
| Protein Phosphatases and Phosphodiesterases Reverse the Action of Cyclic Nucleotide Kinases
|
| There are two ways to terminate a signal initiated by cAMP and cGMP: enhancing degradation of these cyclic nucleotides by phosphodiesterases and dephosphorylation of effectors by protein phosphatases. Phosphodiesterases facilitate the breakdown of cAMP and cGMP to AMP and GMP, respectively, and are activated by ligand activation of GPCRs (Fig. 3-8, B). Phosphatases dephosphorylate effector proteins that were phosphorylated by kinases such as PKA. The balance between kinase-mediated phosphorylation and phosphatase-mediated dephosphorylation allows rapid and exquisite regulation of the phosphorylated state and thus the activity of signaling proteins.
|
| Small, Monomeric G Proteins
|
| page 44 |  | | page 45 |
| Figure 3-9 Arachidonic acid signaling pathways. See text for details. (Redrawn from Boron W, Boulpaep E: Medical Physiology. Philadelphia, Saunders, 2003.) |
| Low-molecular-weight proteins (monomeric G proteins) also play an important role in many signaling pathways. These monomeric G proteins are composed of a single 20- to 40-kDa protein and are membrane bound because of the addition of lipids posttranslationally. Like heterotrimeric G proteins, their activity depends on the binding of GTP, and they are regulated by GEFs and GAPs. Monomeric G proteins have been classified into five families: Ras, Rho, Rab, Ran, and
Arf. Ras GTPases regulate gene expression and cell proliferation, differentiation, and survival. Rho GTPases regulate actin cytoskeletal organization, cell cycle progression, and gene expression. The Rab GTPase family members regulate intravesicular transport and trafficking of proteins between organelles in the secretory and endocytic pathways. Ran GTPases regulate nucleocytoplasmic transport of RNA and proteins. Finally, Arf GTPase, like Rab GTPases, regulate vesicular transport.
|
| Catalytic Receptor-Linked Signal Transduction Pathways
|
| page 45 |  | | page 46 |
| There are two isoforms of cyclooxygenase: COX1 and COX2, the genes for which are located on chromosomes 9 and 1, respectively. COX1 is constitutively expressed. When activated in endothelial cells, COX1 facilitates the production of prostacyclins (Fig. 3-9), which inhibit blood clots (thrombi). COX1 also facilitates the production of thromboxane A2, which is prothrombotic (Fig. 3-9). Thus, cardiovascular health depends in part on the balance between prostacyclins generated by endothelial cells and thromboxane A2, which is produced by vascular smooth muscle cells. COX2 is activated by inflammatory stimuli. Thus, the ability of nonsteroidal antiinflammatory drugs (NSAIDs) (e.g., aspirin, ibuprofen, naproxen, acetaminophen, indomethacin) to suppress the inflammatory response is due to inhibition of COX2. Both COX1 and COX2 facilitate the production of prostanoids that protect the stomach. Recent evidence suggests that both COX1 and COX2 must be inhibited to elicit damage to the gastrointestinal tract. Consequently, the negative effects of NSAIDs on the gastric mucosa (e.g., increased incidence of gastrointestinal bleeding) are most likely due to inhibition of COX1 and COX2 by these nonselective COX inhibitors. However, low doses of aspirin, an NSAID, reduces thromboxane A2 production by platelets with little effect on endothelial prostacyclin production. Thus, low-dose aspirin is antithrombotic. Selective COX2 inhibitors (e.g., celecoxib, rofecoxib, lumiracoxib) are very effective in selectively inhibiting COX2 and are used extensively to reduce the inflammatory response. Because COX2 inhibitors are thought to lack the untoward effects elicited by NSAIDs on the gastrointestinal tract, their use has increased dramatically in the last several years. However, in 2005 the Food and Drug Administration (FDA) announced that COX2-selective drugs are associated with an increased risk for heart attacks and strokes when compared with placebo but not when compared with nonselective NSAIDs. The FDA concluded that both COX2-selective and COX2-nonselective NSAID (COX) inhibitors were associated with an increased risk for adverse cardiovascular events, most likely by inhibiting COX2-mediated prostacyclin production, which as noted earlier is antithrombotic. Subsequently, the FDA required that COX2-selective and COX2-nonselective NSAIDs carry a warning label on product packaging highlighting the potential for the increased risk for adverse cardiovascular events. In addition, although much evidence suggests that COX2-selective inhibitors do not cause gastrointestinal bleeding, recent evidence led the FDA to also require the pharmaceutical industry to add to the warning label on COX2-selective drugs a caution about the potential for increased risk for gastrointestinal bleeding. The cardiovascular risks associated with COX2-selective inhibitors continue to be a topic of debate and intensive research.* |
 |
| Ras GTPases are involved in many signaling pathways that control cell division, proliferation, and death. Many Ras family proteins are oncogenic (cancer causing), whereas others appear to act as tumor suppressors. Mutations in Ras genes that inhibit GTPase activity, as well as overexpression of Ras proteins as a result of transcriptional activation, lead to continuous cell proliferation, a major step in the development of cancer in many organs, including the pancreas, colon, and lung. In addition, mutations in and overexpression of GEFs, which facilitate exchange of GTP for GDP, and GAPs, which accelerate GTP hydrolysis, may also be oncogenic. |
 |
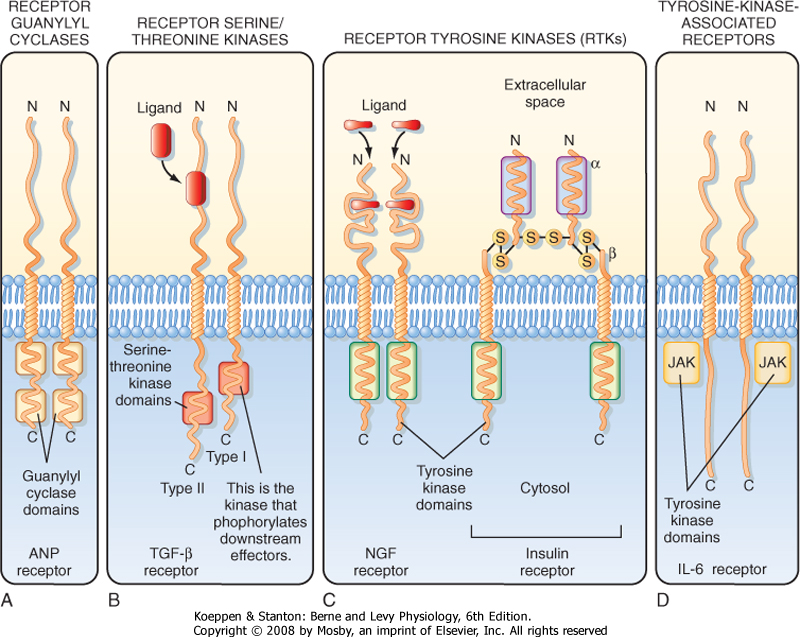
| There are several classes of receptors that have catalytic activity or are intimately associated with proteins that have catalytic activity. Four of these classes will
be discussed, including receptors that mediate the cellular responses to atrial natriuretic peptide (ANP) and NO (receptor guanylyl cyclases); transforming growth factor-β (TGF-β) (receptor threonine/serine kinases); epidermal growth factor (EGF), platelet-derived growth factor (PDGF), and insulin (receptor tyrosine kinases); and interleukins (tyrosine kinase-associated receptors) (Fig. 3-10).
|
| ANP binds to the extracellular domain of the plasma membrane receptor guanylyl cyclase and induces a conformational change in the receptor that causes receptor dimerization and activation of guanylyl cyclase, which metabolizes GTP to cGMP (Fig. 3-10, A). cGMP activates cGMP-dependent protein kinase (PKG), which phosphorylates proteins on specific serine and threonine residues. In the kidney, ANP inhibits sodium and water reabsorption by the collecting duct (see Chapter 34).
|
| NO activates a soluble receptor guanylyl cyclase that converts GTP to cGMP, which relaxes smooth muscle. Because nitroglycerin increases NO production, which increases cGMP and thereby relaxes smooth muscle in coronary arteries, it has long been used to treat angina pectoris (i.e., chest pain caused by inadequate blood flow to heart muscle).
|
| The TGF-β receptor is a threonine/serine kinase that has two subunits (Fig. 3-10, B). Binding of TGF-β to the type II subunit induces it to phosphorylate the type I subunit on specific serine and threonine residues, which in turn phosphorylates other downstream effector proteins on serine and threonine residues and thereby elicits a cellular response.
|
| page 46 |  | | page 47 |
| Figure 3-10 Four types of catalytic receptors are illustrated in this figure. See text for details. (Redrawn from Boron W, Boulpaep E: Medical Physiology. Philadelphia, Saunders, 2003.) |
| There are two classes of tyrosine kinase receptors. Nerve growth factor (NGF) receptors are a typical example of one class (Fig. 3-10, C). Ligand binding to two NGF receptors facilitates their dimerization and activation of tyrosine kinase activity. Activation of the insulin receptor, which is tetrameric and composed of two α and two β subunits, by insulin is an example of the other type of tyrosine kinase receptor. Binding of insulin to the α subunits produces a conformational change that facilitates interaction between the two α and β pairs. Binding of insulin to its receptor causes
autophosphorylation of tyrosine residues in the catalytic domains of the β subunits, and the activated receptor then phosphorylates cytoplasmic proteins to initiate its cellular effects.
|
| The fourth class of catalytic receptors includes the tyrosine-associated receptors, which have no intrinsic kinase activity but associate with proteins that have tyrosine kinase activity, including tyrosine kinases of the Src family and Janus family (JAK) (Fig. 3-10, D). Receptors in this class bind several cytokines, including interleukin-6, and erythropoietin. Tyrosine kinase-associated receptor subunits assemble into homodimers (αα), heterodimers (αβ), or heterotrimers (αβγ) when ligand binds. Subunit assembly enhances the binding of tyrosine kinases, which induces kinase activity and thereby phosphorylates tyrosine residues on the kinases, as well on the receptor.
|
| REGULATION OF GENE EXPRESSION BY SIGNAL TRANSDUCTION PATHWAYS
|
| Steroid and thyroid hormones, cAMP, and receptor tyrosine kinases are transcription factors that regulate gene expression and thereby participate in signal transduction pathways. This section discusses the regulation of gene expression by steroid and thyroid hormones, cAMP, and receptor tyrosine kinases.
|
| Nuclear Receptor-Linked Signal Transduction Pathways
|
| The family of nuclear receptors includes more than 30 genes and has been divided into two subfamilies based on structure and mechanism of action: (1) steroid hormone receptors and (2) receptors that bind retinoic acid, thyroid hormones (iodothyronines), and vitamin D. When ligands bind to these receptors, the ligand-receptor complex activates transcription factors that bind to DNA and regulate the expression of genes (Figs. 3-2, 3-5, and 3-6).
|
| page 47 |  | | page 48 |
The significance of signaling pathways in medicine is illustrated by the following short list of popular drugs that act by regulating signaling pathways.
- Aspirin, the first pharmaceutical (1899), inhibits COX1 and COX2.
- β-Adrenergic receptor agonists and antagonists are used to treat a variety of medical conditions. β1-Agonists increase cardiac contractility and heart rate in patients with low blood pressure. β2-Agonists dilate bronchi and are used to treat asthma and chronic obstructive lung disease. By contrast, β-adrenergic antagonists are used to treat patients with hypertension, angina, cardiac arrhythmias, and congestive heart failure (see Chapter 18).
- Fluoxetine (Prozac) is an antidepressant medication that inhibits reuptake of the neurotransmitter serotonin into the presynaptic cell, which results in enhanced activation of serotonin receptors (see Chapter 6).
- Several monoclonal antibodies are used to treat cancer caused by the activation of growth factor receptors in cancer cells. For example, trastuzumab (Herceptin) is a monoclonal antibody used to treat women with metastatic breast cancer who overexpress HER2/neu, a member of the EGF receptor family that stimulates cell growth and differentiation. Cetuximab (Erbitux) and bevacizumab (Avastin) are monoclonal antibodies that are used to treat metastatic colorectal cancer and head and neck cancer. These antibodies bind to and inhibit the EGF receptor and thereby inhibit EGF-induced cell growth in cancer cells.
- Drugs that inhibit cGMP-specific phosphodiesterase type 5, such as sildenafil (Viagra), Cialis (tadalafil), and Levitra (vardenafil), prolong the vasodilatory effects of NO and are used to treat patients with erectile dysfunction and pulmonary arterial hypertension.
|
 |
| The location of nuclear receptors varies. Glucocorticoid and mineralocorticoid receptors are located in the cytoplasm, where they interact with chaperones (i.e., heat shock proteins) (Fig. 3-2). Binding of hormone to these receptors results in a conformational change that causes chaperones to dissociate from the receptor, thereby uncovering a nuclear localization motif that facilitates translocation of the hormone-bound receptor complex to the nucleus. Estrogen and progesterone receptors are located primarily in the nucleus, and thyroid hormone and retinoic
acid receptors are located in the nucleus bound to DNA (Fig. 3-2).
|
| When activated by hormone binding, nuclear receptors bind to specific DNA sequences in the regulatory regions of responsive genes called hormone response elements. Ligand-receptor binding to DNA causes a conformational change in DNA that initiates transcription. Nuclear receptors also regulate gene expression by acting as transcriptional repressors. For example, glucocorticoids suppress the transcription activator protein-1 (AP-1) and nuclear factor κB (NF-κB), which stimulate the expression of genes that cause inflammation. By this mechanism glucocorticoids reduce inflammation.
|
| As noted previously, cAMP is an important second messenger. In addition to its importance in activating PKA, which phosphorylates specific serine and threonine residues on proteins, cAMP stimulates the transcription of many genes, including those that code for hormones, including somatostatin, glucagon, and vasoactive intestinal polypeptide (Fig. 3-6). Many genes activated by cAMP have a cAMP response element (CRE) in their DNA. Increases in cAMP stimulate PKA, which translocates to the nucleus, where it phosphorylates CREB and thereby increases its affinity for CREB-binding protein (CBP). The CREB-CBP complex activates transcription. The response is terminated when PKA phosphorylates a phosphatase that dephosphorylates CREB.
|
| Many growth factors, including EGF, PDGF, NGF, and insulin, bind to and activate receptors that have tyrosine kinase activity. Activation of tyrosine kinases initiates a cascade of events that enhance the activity of the small GTP-binding protein Ras, which in a series of steps and intermediary proteins leads to transcriptional activation of genes that stimulate cell growth.
|
| Tyrosine kinase-associated receptors, as noted earlier, are activated by a variety of hormones, including cytokines, growth hormone, and interferon. Although these receptors do not have tyrosine kinase activity, they are associated with Janus family proteins (JAK), which do have tyrosine kinase activity. Once activated, hormone tyrosine kinase-associated receptors activate JAK, which phosphorylates latent transcription factors called signal transducers and activators of transcription (STATs). When phosphorylated on tyrosine residues, STATs dimerize and then enter the nucleus and regulate transcription.
|
| page 48 |  | | page 49 |
- The function of cells is tightly coordinated and integrated by external chemical signals, including hormones, neurotransmitters, growth factors, odorants, and products of cellular metabolism, that serve as chemical messengers and provide cell-to-cell communication. Chemical and physical signals interact with receptors located in the plasma membrane, cytoplasm, and nucleus. Interaction of these signals with receptors initiates a cascade of events that mediate the response to each stimulus. These pathways ensure that the cellular response to external signals is specific, amplified, tightly regulated, and coordinated.
- G protein-coupled receptors interact with and regulate ion channels; adenylyl cyclase and the cAMP-PKA signaling pathway; phosphodiesterases, which also regulate cAMP and cGMP signaling pathways; and phospholipases, which regulate the production of prostaglandins, prostacyclins, and thromboxanes. Monomeric G proteins regulate many cellular processes, including gene expression, actin cytoskeleton
organization, cell cycle progression, and intracellular vesicular transport.
- There are four subtypes of catalytic receptors that mediate the cellular response to a wide variety of hormones, including ANP, NO, TGF-β, PDGF, insulin, and interleukins.
- There are two types of nuclear receptors: one type that in the absence of ligand is located in the cytoplasm and when bound to ligand translocates to the nucleus, and another class that permanently resides in the nucleus. Both classes of receptors regulate gene transcription.
|
 |
| page 49 |  | | page 50 |
|