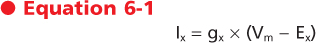| Synaptic transmission is the major process by which electrical signals are transferred between cells within the nervous system (or between neurons and muscle cells or sensory receptors). Within the nervous system, synaptic transmission is usually conceived of as an interaction between two neurons that occurs in a point-to-point manner at specialized junctions called synapses. Two main classes of synapses are distinguished: electrical and chemical. However, as the list of chemical neurotransmitters has grown and as understanding of their mechanisms of action has increased, the definition and conception of what constitutes synaptic transmission has had to be refined and expanded. We no longer think of synaptic transmission as a process that involves only neurons, but now realize that glia form an important element of the synapse and that signaling occurs between neurons and glia. Moreover, in many cases neurotransmitter released at a synapse will act over a widespread territory rather than just at the synapse from which it is released. Thus, we must either generalize the definition of synaptic transmission or consider classically defined synaptic transmission as but one of several mechanisms by which cells in the nervous system communicate with each other. In this chapter we first describe the classic conception of synaptic transmission (electrical and chemical) and then introduce some of the nontraditional neurotransmitters and discuss how they have forced modifications in our conception of chemical communication between cells in the nervous system.
|
| Although their existence in the mammalian central nervous system (CNS) has been known for a long time, electrical synapses, or gap junctions, between neurons were thought to be of relatively little importance in functioning of the adult mammalian CNS. Only recently has it become apparent that these synapses are quite common and that they may underlie important neuronal functions.
|
| An electrical synapse is effectively a low-resistance pathway between cells that allows current to flow directly from one cell to another and, more generally, allows the sharing of small molecules between cells. Electrical synapses are present in the CNS of animals from invertebrates to mammals. They are present between glial cells, as well as between neurons. Electrical coupling of neurons has been demonstrated for most brain regions, including the inferior olive, cerebellum, spinal cord, neocortex, thalamus, hippocampus, olfactory bulb, retina, and striatum.
|
| A gap junction is the morphological correlate of an electrical synapse (see also Chapter 1). These junctions are plaque-like structures in which the plasma membranes of coupled cells become closely apposed (the intercellular space narrows to approximately 3 nm) and filled with electron-dense material (Fig. 6-1). Freeze-fracture electron micrographs of gap junctions display regular arrays of intramembrane particles that correspond to proteins that form the intercellular channels connecting the cells. The typical channel diameter is large (1 to 2 nm), thus making it permeable not only to ions but also other small molecules up to approximately 1 kDa in size.
|
| Each gap junction channel is formed by two hemichannels (called connexons), one contributed by each cell. Each connexon, in turn, is a hexamer of connexin protein subunits, which are encoded for by a gene family of at least 21 different members in mammals. (Recently, a second family of proteins that form gap junctions, the pannexins, has also been identified.) Gap junctions formed by different connexins have distinct biophysical properties (gating and conductance) and cellular distributions. Although at least 10 connexin types are expressed in the CNS, connexin 36 (connexins are named according to their molecular weight; thus, the number refers to the approximate molecular weight of the connexin in kilodaltons) is the major neuronal connexin in the adult CNS. Other connexin types found in the CNS form gap junctions between glial cells or are primarily expressed transiently during development. |
| page 82 |  | | page 83 |
| Figure 6-1 Gap junction structure. A, Schematic view of the gap junction showing narrowing of the intercellular space to 3.5 nm at the junction. The gap junction has multiple channels, with each channel formed by two connexon hemichannels. Each connexon in turn comprises six connexin subunits. B, Electron micrograph of part of a complex synaptic arrangement called a glomerulus that is found in the inferior olive and some other CNS regions. Two dendritic spines are coupled by a gap junction (small black arrows). An axon terminal packed with synaptic vesicles fills the upper right part of the panel. Large arrowheads point to the electron-dense material that marks the active zone. Black dots are immunogold labeling for GABA, thus identifying this terminal as GABAergic. (From De Zeeuw CI, Lang EJ, Sugihara I, et al: J Neurosci 16:3420, 1996. Copyright 1996 by the Society for Neuroscience.) |
| page 83 |  | | page 84 |
| Electrical synapses are fast (essentially no synaptic delay) and bidirectional (i.e., current generated in either cell can flow across the gap junction to influence the other cell). In addition, they act as low-pass filters. That is, slow electrical events are much more readily transmitted than are fast signals such as action potentials. One important role for neuronal gap
junctions appears to be synchronization of network activity. For example, the activity of inferior olivary neurons is normally synchronized but becomes uncorrelated when pharmacological blockers of gap junctions are injected into the inferior olive. It also appears that the patterns of electrical coupling by gap junctions may be highly specific. For example, neocortical interneurons almost exclusively couple to interneurons of the same type. This specific gap junction-coupling pattern suggests that multiple, independent, electrically coupled networks of interneurons may coexist across the neocortex.
|
| Finally, although electrical synapses are generally regarded as relatively simple and static in comparison to chemical synapses, they may actually be fairly dynamic entities. For example, the properties of electrical synapses can be modulated by several factors, including voltage, intracellular pH, and [Ca++]. Moreover, they are subject to regulation by G protein-coupled receptors, and the connexins contain sites for phosphorylation. These factors can change the coupling between cells by causing changes in single-channel conductance, the formation of new gap junctions, or removal of existing ones.
|
| Chemical synaptic transmission was first demonstrated between the vagus nerve and the heart by a simple experiment by Otto Loewi. The vagus nerve of a frog was stimulated to slow the heart rate down while the solution perfusing the heart was collected. This solution was then used to perfuse a second heart, whose beating slowed on being perfused. The chemical responsible was found to be acetylcholine, which we now know is also a neurotransmitter at the neuromuscular junction and at synapses in the peripheral and central nervous systems.
|
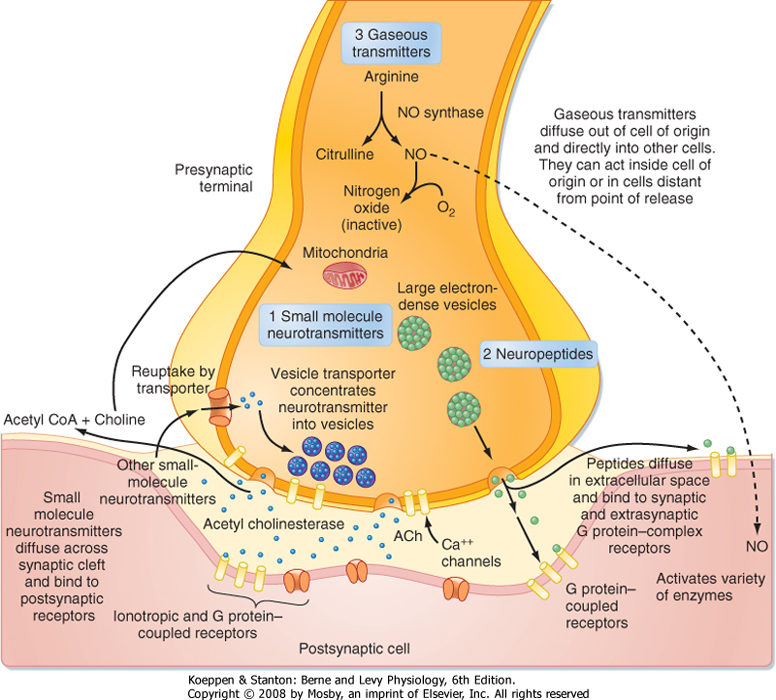
| Figure 6-2 Schematic of a chemical synaptic terminal releasing all three main classes of neurotransmitter. For each, the mechanisms of release, sites of action, and mechanisms for termination of activity are shown. Real synapses release transmitter from one or more classes. |
| page 84 |  | | page 85 |
| Unlike the situation at electrical synapses, at chemical synapses there is no direct communication between the cytoplasm of the two cells. Instead, the cell membranes are separated by a synaptic cleft of some 20 μm, and interaction between the cells occurs via chemical intermediaries known as neurotransmitters. Chemical synapses are generally unidirectional, and thus one can refer to the presynaptic and postsynaptic elements that are diagramed in Figure 6-2. The presynaptic element is often the terminal portion of an axon and is packed with small vesicles whose exact shape and size
vary with the neurotransmitter that they contain. In addition, the presynaptic membrane apposed to the postsynaptic element has regions, known as active zones, of electron-dense material that corresponds to the proteins involved in transmitter release. Moreover, mitochondria and rough endoplasmic reticulum are typically found in the presynaptic terminal. The postsynaptic membrane is also characterized by electron-dense material, which in this case corresponds to the receptors for the neurotransmitter.
|
| Chemical synapses occur between different parts of neurons. Traditionally, focus has been placed on synapses formed by an axon onto the dendrites or soma of a second cell (axodendritic or axosomatic synapses), and our description will be based primarily on such synapses. However, there are many additional types of chemical synapses, such as axoaxonic (axon to axon), dendrodendritic (dendrite to dendrite), and dendrosomatic (dendrite to soma). Furthermore, complex synaptic arrangements are possible, such as mixed synapses, in which cells form both electrical and chemical synapses with each other; serial synapses, in which an axoaxonic synapse is made onto the axon terminal and influences the efficacy of that terminal's synapse with yet a third element; and reciprocal synapses, in which both cells can release transmitter to influence the other. Figure 6-1, B, shows a complex synaptic arrangement, called a glomerulus, that involves both chemical and electrical synapses among the participating elements.
|
| Much of what we know about chemical synapses comes from the study of two classic preparations, the frog neuromuscular junction (the synapse from a motor neuron onto the muscle) and the squid giant synapse (the synapse from a second-order neuron onto third-order neurons that innervate the muscle of the squid's mantle; i.e., the motor neurons, which are the cells whose axons were used to characterize the conductance underlying the action potential [see Chapter 5]). The principles governing transmission at these synapses mostly apply to synapses within the mammalian CNS as well, at least with regard to synapses using what are called the "classic" neurotransmitters (see the section Neurotransmitters). Thus, much of the following discussion will be based on results from these two preparations; however, some differences in CNS synapses will also be pointed out.
|
|
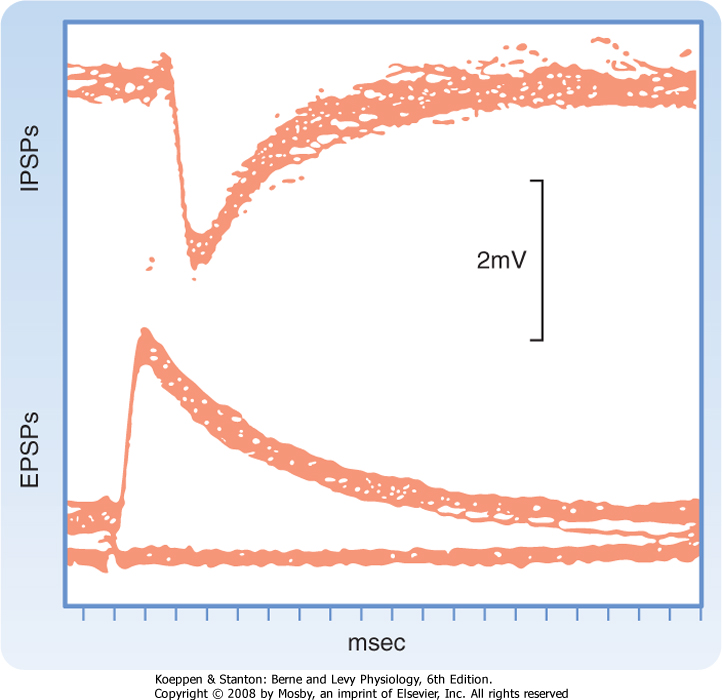
| Figure 6-3 IPSPs and EPSPs recorded with a microelectrode in a cat spinal motor neuron in response to stimulation of appropriate peripheral afferent fibers. Forty traces are super-imposed. Note that these IPSPs are hyperpolarizing, but in some cases IPSPs can be depolarizing-see text for an explanation. (Redrawn from Curtis DR, Eccles JC: J Physiol 145:529, 1959.) |
| Synaptic transmission at a chemical synapse may be summarized as follows. Synaptic transmission is initiated by arrival of the action potential at the presynaptic terminal. The action potential depolarizes the terminal, which causes Ca++ channels to open. The subsequent rise in [Ca++] within the terminal triggers the fusion of vesicles containing neurotransmitter with the plasma membrane. The transmitter is then expelled into the synaptic cleft, diffuses across it, and binds to specific receptors on the postsynaptic membrane. Binding of transmitter to receptors then causes the opening (or less often, the closing) of ion channels in the postsynaptic membrane, which in turn results in changes in the potential and resistance of the postsynaptic membrane that alter the excitability of the
cell. The changes in membrane potential of the postsynaptic cell are termed excitatory and inhibitory postsynaptic potentials (EPSPs and IPSPs) (Fig. 6-3), depending on whether they increase or decrease, respectively, the cell's excitability, which can be defined as its probability of firing action potentials. The transmitter acts for only a very short time (milliseconds) because reuptake and degradation mechanisms rapidly clear the transmitter from the synaptic cleft.
|
| The succeeding sections will amplify specific points of this summary. However, it is worth mentioning at this point that some of the nonclassic types of neurotransmitters (e.g., neuropeptides and gaseous neurotransmitters such as nitric oxide) and the discovery of metabotropic receptors have required modifications of several aspects of this basic conception (a metabotropic receptor does not contain an ion channel but, instead, is coupled to a G protein that initiates second messenger cascades that ultimately affect ion channels, whereas an ionotropic receptor contains the ion channel as an integral part of itself). Some of the differences between classic and peptide transmitters are listed in Table 6-1. More details on the properties of peptide and gaseous transmitters are provided in the relevant parts of the Neurotransmitters section of this chapter, and metabotropic receptors are covered in the Receptors section.
|
| Calcium Entry Is the Signal for Transmitter Release
|
| page 85 |  | | page 86 |
|
Table 6-1.
Distinctions between Classic Nonpeptide Neurotransmitters and Peptide Neurotransmitters |
| Nonpeptide Transmitters | Peptide Transmitters |
| Synthesized and packaged in the nerve terminal | Synthesized and packaged in the cell body; transported to the nerve terminal by fast axonal transport |
| Synthesized in active form | Active peptide formed when it is cleaved from a much larger polypeptide that contains several neuropeptides |
| Usually present in small, clear vesicles | Usually present in large, electron-dense vesicles |
| Released into a synaptic cleft | May be released some distance from the postsynaptic cell There may be no well-defined synaptic structure |
| Action of many terminated because of uptake by presynaptic terminals via Na+-powered active transport | Action terminated by proteolysis or by the peptide diffusing away |
| Typically, action has short latency and short duration (msec) | Action may have long latency and may persist for many seconds |
| Depolarization of the presynaptic membrane by the action potential causes voltage-gated Ca++ channels to
open, which makes it possible for Ca++ to flow into the terminal and trigger the release of transmitter. However, Ca++ will enter the terminal only if there is a favorable electrochemical gradient to do so. Recall that it is the combination of the concentration and voltage gradients that determines the direction of ion flow through open channels. Extracellular [Ca++] is high relative to intracellular [Ca++], which favors entry into the terminal; however, during the peak of the action potential, the membrane potential is positive, and the voltage gradient opposes the entry of Ca++ because of its positive charge. Thus, at the peak of the action potential, relatively little Ca++ enters the terminal because although the membrane is highly permeable to Ca++, the overall driving force is small. In fact, by using a voltage clamp, one can experimentally make the membrane potential positive and equal to the Nernst equilibrium potential for Ca++. If this is done, no Ca++ will enter the terminal despite Ca++ channels being open, and as a result no transmitter is released and no postsynaptic response is observed. This voltage is known as the suppression potential. If the membrane potential is rapidly made negative again (because of either the end of the action potential or by adjusting the voltage clamp), Ca++ rushes into the terminal as a result of the large driving force (which arises instantaneously on repolarization) and the high membrane permeability to Ca++ (which remains high because it takes the Ca++ channels several milliseconds to close in response to the new membrane potential), thereby resulting in release of transmitter and a postsynaptic response (Fig. 6-4).
|
| Synaptic Vesicles and the Quantal Nature of Transmitter Release
|
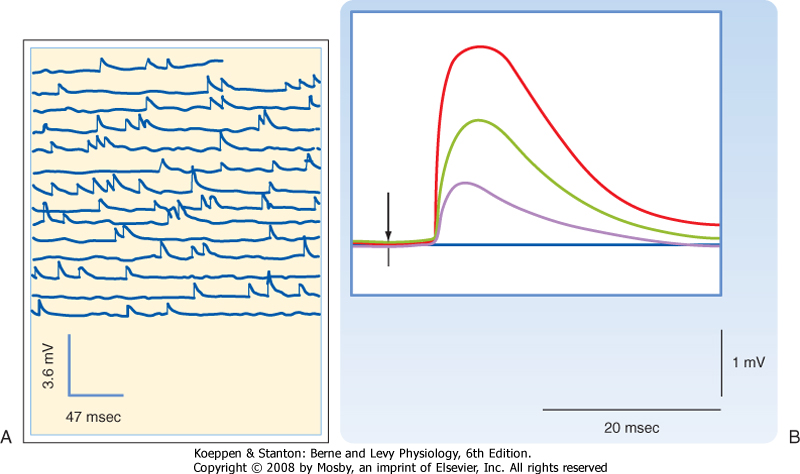
| How neurotransmitter is stored and how it is released are questions fundamental to synaptic transmission. Answering these questions began with two observations. The first was the discovery of small round or irregularly shaped organelles known as synaptic vesicles in presynaptic terminals by electron microscopy (Fig. 6-2). The second observation came from recordings of postsynaptic responses at the neuromuscular junction. Normally, an action potential in a motor neuron causes a large depolarization in the postsynaptic muscle, termed an end plate potential (EPP), which is equivalent to an EPSP in a neuron. However, under conditions of low extracellular [Ca++], the EPP amplitude is reduced (because the presynaptic Ca++ current is reduced, leading to a smaller rise in intracellular [Ca++], transmitter release proportional to [Ca++]). In this condition, the EPP is seen to fluctuate among discrete values (Fig. 6-5). Moreover, small, spontaneous depolarizations of the postsynaptic membrane, termed miniature end plate potentials (mEPPs), are observable. The amplitude of the mEPP (≤1 mV) corresponds to that of the smallest EPP evoked under low [Ca++], and the amplitudes of other EPPs were shown to be integral multiples of the mEPP amplitude; thus, it was natural to propose that each mEPP corresponded to the release of transmitter from a single vesicle and that EPPs represented the combined simultaneous release of transmitter from many vesicles.
|
| This linking of mEPPs and vesicles implies that each mEPP is caused by the action of many molecules of neurotransmitter binding to postsynaptic receptors. The alternative that each mEPP could be caused by a single transmitter molecule binding to and opening a single postsynaptic receptor was rejected, in part because responses smaller in amplitude than mEPPs could be generated experimentally by directly applying dilute solutions of acetylcholine to the muscle. In fact, mEPPs were calculated to be caused by the action of approximately 10,000 molecules, which corresponds well to estimates of the number of neurotransmitter molecules contained within a single vesicle.
|
| Many additional studies have confirmed the vesicle hypothesis of neurotransmitter release. For example, biochemical studies have shown that neurotransmitter is concentrated in vesicles, and fusion of vesicles to the plasma membrane and their depletion in the terminal cytoplasm after action potentials have been shown with electron microscopic techniques.
|
| Molecular Apparatus Underlying Vesicular Release
|
| page 86 |  | | page 87 |
|
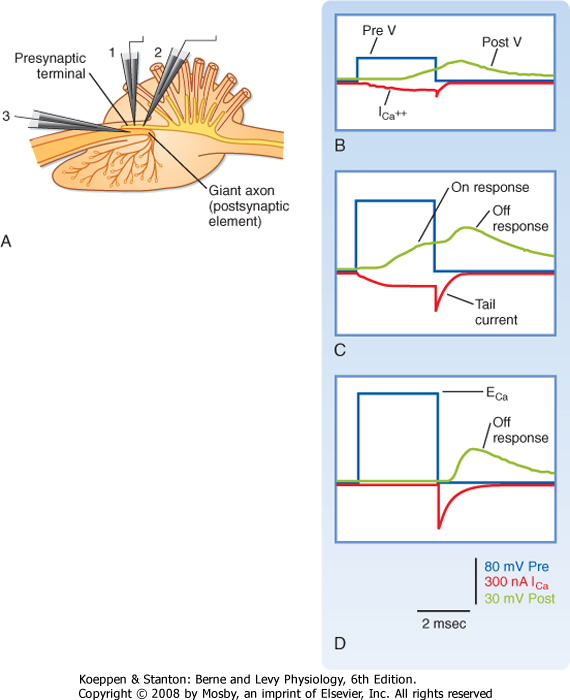
| Figure 6-4 Presynaptic Ca++ current and its relationship to the postsynaptic response. A, Schematic of a squid giant synapse preparation. Electrodes 1 and 2 are used to voltage-clamp the presynaptic terminal and record its voltage and current. Note that TTX and TEA were present to block Na+ and K+ conductance, in order to isolate the Ca++ conductance. Electrode 3 records the membrane potential of the postsynaptic axon. The presynaptic terminal was voltage-clamped to increasingly more depolarized levels (blue traces). With a small depolarization (B), a small Ca++ current starts shortly after the voltage step, continues to grow for the duration of the step (on current), and then decays exponentially after its termination (off or tail current). A larger voltage step (C) increases both the on and the off components of the Ca++ current, and now distinct on and off responses are observed in the postsynaptic response. D, The voltage step is to the Nernst potential for Ca++, so there is no Ca++ current during the step, but a large tail current and off response are observed. Based on data of Llinas R, et al: Biophys J. 33:323-351, 1981. TTX-tetrodotoxin; TEA, tetraethyl ammonium. |
|
| Figure 6-5 A, Spontaneous mEPPs recorded at a neuromuscular junction in a fiber of frog extensor digitorum longus. B, EPPs evoked by nerve stimulation under low-[Ca++] conditions, which reduce the probability of transmitter release. The small-amplitude EPPs evoked under these conditions vary in amplitude in a step-like manner, where the size of the step is equal to the smallest EPP, which in turn equals the size of the mEPPs (note that in these conditions the stimulus often fails to evoke any response, as indicated by a flat response). (A, Data from Fatt P, Katz B: Nature 166:597, 1950; B, data from Fatt P, Katz B: J Physiol 117:109, 1952.) |
| page 87 |  | | page 88 |
| The small vesicles that contain nonpeptide neurotransmitters can fuse with the presynaptic membrane only at specific sites, called active zones. To become competent to fuse with the presynaptic membrane at an active zone, a small vesicle must first dock at the active zone. It must then undergo a priming process
before the vesicle can fuse and release its transmitter into the synaptic cleft in response to an increase in local cytoplasmic [Ca++]. On the order of 25 proteins may play roles in docking, priming, and fusion. Some of these proteins are cytosolic, whereas others are proteins of the vesicle membrane or the presynaptic plasma membrane. The functions of most of these proteins are incompletely understood; however, knowledge of the molecular details of transmitter release has increased dramatically in recent years.
|
| As with other exocytotic processes, neurotransmitter release involves SNARE proteins: v-SNARES in the vesicle membrane and t-SNARES on the (target) presynaptic plasma membrane. Zipper-like interactions between synaptobrevin (a v-SNARE) and syntaxin and SNAP-25 (two t-SNARES) bring the vesicle membrane and the presynaptic plasma membrane close together before fusion. The SNARE proteins are targets for various botulinum toxins, which disrupt synaptic transmission, thus demonstrating their critical role in this process. Nevertheless, they do not bind Ca++, so another protein must be the Ca++ sensor that triggers the actual fusion event. Although several proteins in the terminal do bind Ca++, synaptotagmin is almost certainly the Ca++ sensor.
|
| Calcium channels are located in the active zone membrane at sites adjacent to the docked vesicles. When they open, a small area of high [Ca++], which lasts for less than a millisecond and is termed a microdomain, is created at the active zone. This local high concentration allows the rapid binding of Ca++ to a protein called synaptotagmin, and it is thought that this binding causes a conformational change in synaptotagmin that triggers the fusion event of a docked vesicle. Indeed, the time from Ca++ influx to vesicle fusion is about 0.2 msec.
|
| Synaptic Vesicles Are Recycled
|
| During synaptic transmission, vesicles must fuse with the plasma membrane to release their contents into the synaptic cleft. However, there must be a reverse process; otherwise, not only would it be hard to sustain the vesicle population, but the presynaptic membrane's surface area would also grow with each bout of synaptic transmission, and its molecular content and functionality would likewise change (because, as just discussed, the protein content of the vesicle membrane is distinct from that of the terminal membrane).
|
|
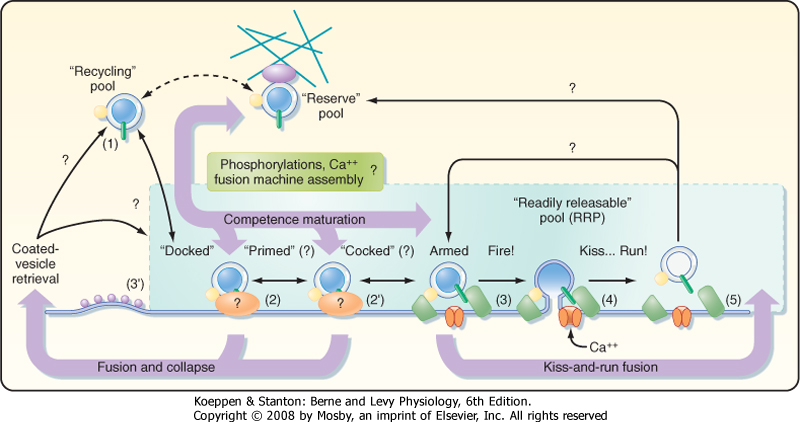
| Figure 6-6 Vesicle recycling pathways. Synaptic vesicles have been thought to fuse with the membrane while emptying their contents and then be recycled by forming clathrin-coated pits that are endocytosed to form coated vesicles (1 → [2 or 2'] → 3' → 1). An alternative pathway that may allow more rapid recycling of vesicles has been proposed. This pathway, called "kiss and run," involves only transient fusion of the vesicle to the presynaptic membrane to form a pore through which the vesicle contents may be emptied, followed by detachment of the vesicle from the membrane (1 → 2 → 3 → 4 → 5 → 1). (Redrawn from Valtorta F, Meldolesi J, Fesce R: Trends Cell Biol 11:324, 2001.) |
| page 88 |  | | page 89 |
| There appear to be two distinct mechanisms by which vesicles are retrieved after release of their neurotransmitter content (Fig. 6-6). One mechanism is the endocytotic pathway commonly found in most cell types. Coated pits are formed in the plasma membrane, which then pinch off to form coated vesicles within the cytoplasm of the presynaptic terminal. These vesicles then lose their coat and undergo further transformations (i.e., acquire the correct complement
of membrane proteins and be refilled with neurotransmitter) to become once again synaptic vesicles ready for release.
|
| Recently, evidence for a second, more rapid recycling mechanism has been obtained (Fig. 6-6). It involves transient fusion of the vesicle to the synaptic membrane and has been called "kiss and run." In this case, fusion of the vesicle with the synaptic membrane leads to the formation of a pore through which the transmitter is expelled, but there is no wholesale collapse of the vesicle into the membrane. Instead, the duration of the fusion is very brief, after which the vesicle detaches from the plasma membrane and reseals itself. Thus, the vesicle membrane retains its molecular identity. Its contents can then simply be replenished, thereby making the vesicle ready for use again.
|
| The relative importance of these two mechanisms is still being debated. However, at central synapses, which tend to be small and contain relatively few vesicles in comparison to the neuromuscular junction, the rapid time course of the kiss-and-run mechanism may help avoid the problem of vesicle depletion and the consequent failure of synaptic transmission during periods of high activity (many neurons in the CNS can show sustained firing rates of several hundred hertz, and a few types of neuron can fire at rates of approximately 1000 Hz).
|
| When an action potential triggers release of neurotransmitter from a motor neuron, an EPP is generated in the muscle. More generally, at excitatory synapses throughout the nervous system, action potentials trigger EPSPs in the postsynaptic cell. In both cases there is a depolarization of the membrane that increases the excitability of the cell (i.e., makes it more likely to fire an action potential or, if it is already active, increases the firing rate). The EPP is so large that under normal circumstances it depolarizes the sarcolemma well above the action potential threshold and thus always triggers a spike leading to contraction of the muscle cell. This is an example of a synapse with a high (>1) safety factor (ratio of synaptic potential to the amplitude needed to reach threshold), which makes sense for the neuromuscular junction because each muscle cell is contacted by only a single motor neuron and if that motor neuron is firing, the nervous system has basically made the decision to contract that muscle. In contrast, most neurons receive thousands of excitatory synapses from many different cells. Here, each synapse generates a small EPSP, and thus it takes the summed EPSPs of multiple active synapses to trigger an action potential in the postsynaptic neuron.
|
| In both situations the basic process leading to the EPSP is the same: the neurotransmitter binds to receptors in the postsynaptic cell that open channels to allow an inward current to flow, which in turn leads to depolarization of the membrane. These channels are termed ligand gated because their opening and closing is primarily controlled by the binding of neurotransmitter. This mechanism can be contrasted with the channels underlying the action potential, which open and close in response to changes in membrane potential. However, there are some channels, most notably the NMDA (N-methyl-d-aspartate) channel, that are both ligand and voltage gated.
|
| It is also worth noting here that the preceding description and what follows in this section refer to what happens when neurotransmitter binds to receptors in which the ion channel is part of the receptor itself. These receptors are referred to as ionotropic receptors and underlie what is now called "fast" synaptic transmission. There is also "slow" synaptic transmission, mediated by what are called metabotropic receptors, in which the receptor and ion channel are not part of the same molecule and binding of neurotransmitter to the receptor initiates biochemical cascades that lead to much slower responses (see the section Receptors for details). Despite the differing time courses, many of the same basic principles apply to both types of synaptic potential.
|
Once the EPSP channels are open, the direction of current flow through them is determined by the electrochemical driving force for the permeant ions. It turns out that the pores of most channels that underlie EPSPs are relatively large and therefore allow passage of most cations with similar ease. As an example, consider the acetylcholine-gated channel that is opened at the neuromuscular junction. Na+ and K+ are the major cations present (Na+ extracellularly and K+ intracellularly); therefore, the net current through the channel is approximately the sum of the Na+ and K+ currents (Inet = INa + IK). Recall that the current through a channel from a particular ion is dependent on two factors: the conductance of the channel to the ion and the driving force on the ion. This relationship is expressed by the equation
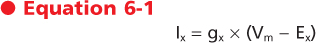
where gx is the conductance of the channel to ion x, Vm is the membrane potential, and Ex is the Nernst equilibrium potential for ion x. In this case gx is similar for Na+ and K+, so the main determinant of net current is the relative driving forces (Vm - Ex). If the membrane is at its resting potential (typically around -70 mV), there is a strong driving force (Vm - ENa) for Na+ to enter the cell because this potential is far from the Na+ Nernst potential (about +55 mV), whereas there is only a small driving force for K+ to leave the cell because Vm is close to the K+ Nernst potential (about -90 mV). Thus, if acetylcholine-gated channels open when the membrane is at its resting potential, a large inward Na+ current and a small outward K+ current will flow through the acetylcholine channel, thereby resulting in a net inward current, which acts to depolarize the membrane.
|
| page 89 |  | | page 90 |
| Figure 6-7 Properties of EPSPs. A, Time course of a fast EPSP compared with that of the underlying EPSC. In many cases, such as this one, the EPSC is much shorter than the EPSP; however, sometimes the EPSC can have a fairly extensive tail. B, Intracellularly recorded EPSPs at different levels of depolarization. EPSPs were evoked in motor neurons by stimulation of Ia afferents. The number to left of each trace indicates the membrane potential induced by injection of current through the electrode. At initial membrane potentials of -42 and -60 mV, the EPSP triggered an action potential. At more depolarized levels, Na+ channels are inactivated, so no spike occurs. C, To determine the EPSP reversal potential, the initial membrane potential is plotted against the size of the EPSP (ΔV). This EPSP reversed at -7 mV. (A, Data from Curtis DR, Eccles JC: J Physiol 145:529, 1959; B, data from Coombs JS et al: J Physiol 130:374, 1955.) |
| The net inward current that results from opening such channels is called the excitatory postsynaptic current (EPSC). Figure 6-7, A, contrasts the time course of the EPSC and the resulting EPSP for fast synaptic transmission. The EPSC is much shorter (≈1 to 2 msec
in duration) and corresponds to the time that the channels are actually open. The short duration of the EPSC is due to the fact that the released neurotransmitter remains in the synaptic cleft for only a short while before being either enzymatically degraded or taken up by either glia or the presynaptic terminal. Binding and unbinding of a neurotransmitter to its receptor take place rapidly, so once its concentration falls in the cleft, the postsynaptic receptor channels rapidly close as well and terminate the EPSC. Note how the end of the EPSC corresponds to the peak of the EPSP, which is followed by a long tail. The duration of the tail and the rate of the decay in EPSP amplitude reflect the passive membrane properties of the cell (i.e., its RC properties). In slow synaptic transmission, the duration of the EPSP reflects the activation and deactivation of biochemical processes more than the membrane properties. The long duration of even fast EPSPs is functionally important because it allows EPSPs to overlap and thereby summate. Such summation is central to the integrative properties of neurons (see the later section Synaptic Integration).
|
| page 90 |  | | page 91 |
| Normally, an EPSP depolarizes the membrane, and if this depolarization reaches threshold, an action potential is generated. However, consider what happens if the channels underlying the action potential are blocked and the membrane of the postsynaptic cell is experimentally depolarized by injecting current through an intracellular electrode. Because the membrane potential is now more positive, the driving force for Na+ is decreased and that for K+ increased. If the synapse is activated at this point, the net current through the receptor channel (the EPSC) will be smaller because of changes in the relative driving force. This implies that if the membrane potential is depolarized enough, there will be a point at which the Na+ and K+ currents through the channel are equal and opposite and thus there is no net current and no EPSP. If the membrane is depolarized beyond this point, there is a net outward current through the receptor channels, and the membrane will hyperpolarize (i.e., the EPSP will be negative). Thus, the potential at which there is no EPSP (or EPSC) is known as the reversal
potential. For excitatory synapses, the reversal potential is usually around 0 mV (±10 mV), depending on the synapse (Fig. 6-7, B and C).
|
| It is worth noting that a reversal potential is a key criterion for demonstrating the chemical gated as opposed to the voltage-gated nature of a synaptic response because currents through voltage-gated channels do not reverse, except at the Nernst potential of the ion for which they are selective (and then only if the channel is open at that potential). Consequently, beyond a certain membrane potential, no current will flow through voltage-gated channels because they will be closed. In contrast, ligand-gated channels remain open regardless of the membrane potential and thus will always allow current flow, except at one specific voltage, the reversal potential.
|
| IPSPs, like EPSPs, are triggered by the binding of neurotransmitter to receptors on the postsynaptic membrane and typically involve an increase in membrane permeability as a result of the opening of ligand-gated channels. They differ in that IPSP channels are permeable to only a single ionic species, either Cl- or K+. Thus, IPSPs will have a reversal potential equal to the Nernst potential of the ion carrying the underlying current. Typically, the Nernst potential for these ions is somewhat negative relative to the resting potential, so when IPSP channels open, there is an outward flow of current through them that results in hyperpolarization of the membrane.
|
| However, in some cells, activation of an inhibitory synapse may produce no change in potential (if the membrane potential equals the Nernst potential for Cl- or K+) or may actually result in a small depolarization. Nevertheless, in both these cases, the reversal potential for the IPSP is still negative with regard to the threshold for eliciting an action potential (otherwise it would increase the probability of the cell spiking and by definition be an EPSP). It may seem counterintuitive that something that depolarizes the membrane can still be considered inhibitory, but if it decreases the probability of spiking, then it is indeed inhibitory. A further explanation is given in the next section.
|
| In sum, starting from the resting membrane potential, EPSPs are always depolarizing, IPSPs can be either depolarizing or hyperpolarizing, and a hyperpolarizing potential is always an IPSP. Thus, the key distinction between inhibitory and excitatory synapses (and IPSPs and EPSPs) is how they affect the probability of the cell firing an action potential: EPSPs increase the probability, whereas IPSPs decrease the probability.
|
| The overall effect of a particular synapse is dependent on its location. To understand this concept fully, we must first recall that action potentials are typically generated at the axon hillock of the cell because it has the highest density of voltage-gated Na+ channels and therefore the lowest threshold for initiation of a spike. Thus, it is the summed amplitudes of the synaptic potentials at this point, the axon hillock, that is critical for the decision to spike. EPSPs generated by synapses close to the axon hillock (i.e., synapses onto the soma or proximal dendrites) will result in a larger depolarization at the hillock than will EPSPs generated by synapses on distal dendrites (Fig. 6-8, A, single action potential in axon 1 versus 2). Remember that the cell membrane is leaky and synaptic currents are generated locally at the synapse, so even if two synapses generate a local EPSC of the same size, less of the initial current will arrive at the axon hillock from the more distal synapse than from the more proximal one, thereby resulting in the generation of a smaller EPSP at the axon hillock by the distal synapse (see discussion of length constant in Chapter 5). Thus, the synapse's spatial location in the dendritic tree is an important determinant of its efficacy.
|
|
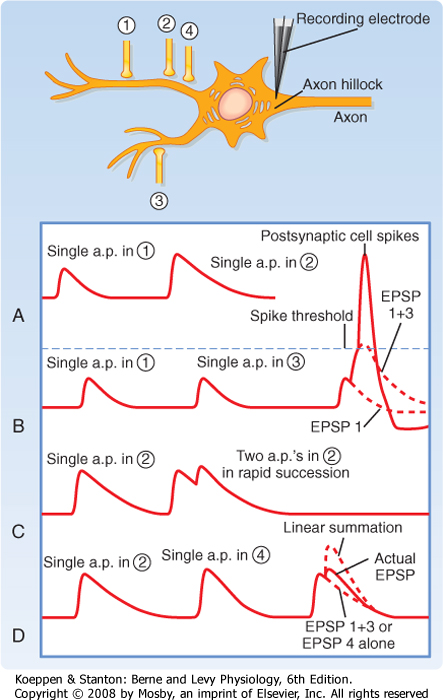
| Figure 6-8 Synaptic integration of EPSPs recorded at axon hillock. A, Comparison of EPSPs evoked by proximal versus distal synapses (1 versus 2). B, Example of a spatial summation response evoked by synapses that are electrically independent of each other (1 and 3). C, Temporal summation. The postsynaptic response to two spikes in the same axon occurs in rapid succession (axon 2). D, Sublinear summation of two synapses located near each other (2 and 4). a.p., action potential. |
| page 91 |  | | page 92 |
| As already mentioned, EPSPs generated by most CNS synapses, even those in favorable positions (i.e., close to the axon hillock), are too small by themselves
to reach the spiking threshold in the postsynaptic cell. An action potential will result only when the summed excitation from multiple inputs reaches threshold. For example, in Figure 6-8, suppose axon 1 fires an action potential. This results in an EPSP that depolarizes the cell but is too small to reach threshold. Now suppose that axon 1 fires an action potential followed by an action potential in axon 3 a short time later. Each of the resulting EPSPs is too small to reach threshold, but if they occur within a short time of each other, their effect can be additive as shown in Figure 6-8, B. The combined amplitude may then reach threshold and lead to spiking of the cell. The ability of such asynchronous EPSPs to summate is known as temporal summation. The fact that EPSPs have a long time course (when compared with action potentials or the underlying EPSCs) facilitates this type of synaptic integration. Temporal summation can also occur when the same synapse is activated multiple times in rapid succession because axons can fire action potentials at rates well over 100 Hz; in this situation, successive EPSPs will be less than 10 msec apart and therefore overlap and summate (Fig. 6-8, C).
|
| The example of temporal summation between two synapses just presented also illustrates the principle of spatial summation, which refers to the fact that synaptic potentials generated throughout the soma and dendrites interact. Interestingly, the nature of this interaction depends on the relative locations of the two synapses. In the foregoing example, the combined EPSP was approximately the linear summation of the two individual EPSPs evoked by action potentials in axons 1 and 3. This is the case when two synapses are far apart. If the two synapses are close together, such as for axons 2 and 4 (Fig. 6-8, D), the summation becomes less than linear because of what is known as a shunting effect. That is, when synapse 2 is active, channels are opened in the cell membrane, which means that it is more leaky. Therefore, when synapse 4 is also active, more of its EPSC will be lost (shunted) through the dendritic membrane, and less current will be left to travel down the dendrite to the axon hillock. The result is that synapse 4 causes a smaller EPSP at the hillock than it would have generated in isolation. Nevertheless, the combined EPSP is still larger than an EPSP caused by either synapse 2 or 4 alone.
|
| Where do IPSPs fit into synaptic integration? In many cases one can think of them as negative EPSPs. Thus, whereas EPSPs add together to help bring the membrane potential up to and beyond the spiking threshold, IPSPs subtract from the membrane potential to make it more negative and therefore further from threshold. In deciding whether to spike, a cell adds up the ongoing EPSPs and subtracts the IPSPS to determine whether the sum reaches threshold. As with an EPSP, the efficacy of an IPSP varies with its location.
|
| In addition to subtracting algebraically from the membrane potential, IPSPs exert an inhibitory action via the shunting mechanism, just as was described earlier for EPSPs. That is, while the IPSP channels are open, they make the membrane very leaky (i.e., lower its resistance) and thereby reduce the size of EPSPs, thus making them less effective. This shunting mechanism explains how IPSPs that do not change the membrane potential-or even those that slightly depolarize it-can still decrease the excitability of the cell. An alternative way to look at this effect is to view each synapse as a device that tries to bring the membrane potential to its own equilibrium potential. Because this potential is below the action potential threshold in the case of IPSPs, it makes it harder for the cell to spike.
|
| Thus far the interaction of synaptic potentials has been presented under the assumption that the postsynaptic cell membrane is passive (i.e., it acts as though it were simply resistors and capacitors in parallel with each other). However, recent evidence has made it clear that the dendrites and somas of most, if not all, neurons contain active elements (i.e., gated channels) that can amplify and alter EPSPs and IPSPs. For example, distal EPSPs can have a larger than expected effect because of voltage-gated Na+ or Ca++ channels that are activated by the EPSP and that, in turn, boost its amplitude or even underlie propagated dendritic action potentials. Another example is Ca++-activated K+ channels that are present in the dendrites of some neurons. These channels are activated by the influx of Ca++ either through synaptic channels or via dendritic voltage-gated Ca++ channels opened by EPSPs and can cause long-lasting hyperpolarizations that effectively make the cell inexcitable for tens to hundreds of milliseconds. As a final example, there are some Ca++ channels that underlie a low-threshold Ca++ spike. These channels are normally inactive at resting membrane potentials, but the hyperpolarization that results from a large IPSP can de-inactivate them and allow them to open (and produce a spike) after termination of the IPSP. In this case "inhibition" actually increases the cell's excitability. In sum, synaptic integration is a highly complex, nonlinear process. Nevertheless, the basic principles just described remain at its core.
|
| MODULATION OF SYNAPTIC ACTIVITY
|
| Integration of synaptic input by a postsynaptic neuron, as described in the previous section, represents one aspect of the dynamic nature of synaptic transmission. A second dynamic aspect is that the strength of individual synapses can vary as a function of their use or activity. That is, a synapse's current functional state reflects, to some extent, its history.
|
| page 92 |  | | page 93 |
| Activation of a synapse typically produces a response in the postsynaptic cell (i.e., a postsynaptic potential) that will be roughly the same each time, assuming that the postsynaptic cell is in a similar state. Certain patterns of synaptic activation, however, result in changes in the response to subsequent activation of the synapse. Such use-related changes may remain for short (milliseconds) or long (minutes to days) durations and may be either a potentiation or suppression of the synapse's strength. These changes
probably underlie cognitive abilities, such as learning and memory. Thus, the processes by which activity results in changes in a synapse's efficacy are a critical feature of synaptic transmission.
|
| Paired-Pulse Facilitation
|
| When a presynaptic axon is stimulated twice in rapid succession, it is often found that the response evoked by the second stimulus is larger in amplitude than the one evoked by the first (Fig. 6-9). This increase is known as paired-pulse facilitation (PPF). If one plots the relative size of the two postsynaptic potentials (PSPs) (i.e., the responses) as a function of the time between two stimuli, the amount of increase in the second PSP will be seen to depend on the time interval. Maximal facilitation occurs at around 20 msec, followed by a gradual reduction in facilitation as the interstimulus interval continues to increase; with intervals of several hundred milliseconds, the two PSPs are equal in amplitude and no facilitation is observed. Thus, PPF is a relatively rapid, but short-lasting change in synaptic efficacy.
|
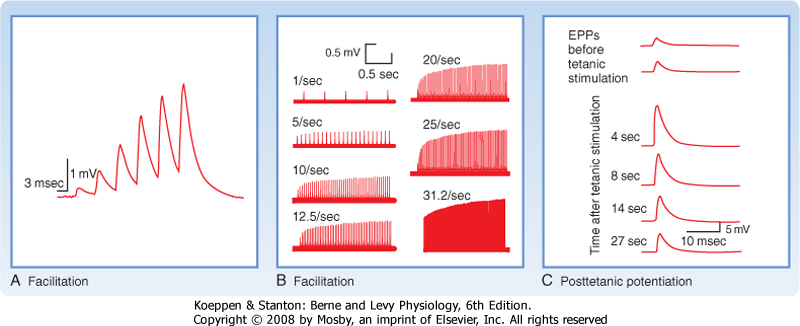
|
| Figure 6-9 A, Facilitation at a neuromuscular junction. EPPs at a neuromuscular junction in toad sartorius muscle were elicited by successive action potentials in the motor axon. Neuromuscular transmission was depressed by 5 mM Mg++ and 2.1 mM curare so that action potentials did not occur. B, EPPs at a frog neuromuscular junction elicited by repetitively stimulating the motor axon at different frequencies. Note that facilitation failed to occur at the lowest frequency of stimulation (1/sec) and that the degree of facilitation increased with increasing frequency of stimulation in the range of frequency used. Neuromuscular transmission was inhibited by bathing the preparation in 12 to 20 mM Mg++. C, Posttetanic potentiation at a frog neuromuscular junction. The top two traces indicate control EPPs in response to single action potentials in the motor axon. Subsequent traces indicate EPPs in response to single action potentials after tetanic stimulation (50 impulses/sec for 20 seconds) of the motor neuron. The time interval between the end of tetanic stimulation and the single action potential is shown on each trace. The muscle was treated with tetrodotoxin to prevent the generation of action potentials. (A, Redrawn from Belnave RJ, Gage PW: J Physiol 266:435, 1977; B, Redrawn from Magelby KL: J Physiol 234:327, 1973; C, redrawn from Weinrich D: J Physiol 212:431, 1971.) |
| Posttetanic potentiation is similar to PPF; however, in this case the responses are compared before and after stimulation of the presynaptic neuron tetanically (tens to hundreds of stimuli at a high frequency). Such a tetanic stimulus train causes an increase in synaptic
efficacy, known as posttetanic potentiation (Fig. 6-9, C). Posttetanic potentiation, like facilitation, is an enhancement of the postsynaptic response, but it lasts longer (Fig. 6-9, C): tens of seconds to several minutes after the cessation of tetanic stimulation.
|
| Numerous experiments have shown that PPF and posttetanic potentiation are the result of changes in the presynaptic terminal and do not generally involve a change in the sensitivity of the postsynaptic cell to transmitter. Rather, the repeated stimulation leads to an increased number of quanta of transmitter being released. This increase is thought to be due to residual amounts of Ca++ that remain in the presynaptic terminal after each stimulus and help potentiate subsequent release of transmitter. However, the exact mechanism or mechanisms by which this residual Ca++ enhances release is not yet clear. The residual Ca++ does not, however, appear to act simply by binding to the same sites as the Ca++ that enters at the active zone and directly triggers vesicle fusion in response to the action potential.
|
| page 93 |  | | page 94 |
| Use of a synapse can also lead to a short-term depression in its efficacy. Most commonly, the postsynaptic cell at such a fatigued or depressed synapse responds normally to transmitter applied from a micropipette; hence, as was the case for PPF and posttetatanic
potentiation, the change is presynaptic. In general, the depression is thought to reflect depletion of the number of releasable presynaptic vesicles. Thus, short-term depression of synaptic transmission is most often and most easily seen at synapses in which the probability of release after a single stimulus is high and under conditions that favor release (i.e., high [Ca++]). A postsynaptically related cause of synaptic depression can be desensitization of the receptors in the postsynaptic membrane.
|
| Both potentiation and depressive processes can occur at the same synapse. So in general, the type of modulation observed will depend on which process dominates. This, in turn, can reflect stimulus parameters, local ionic conditions, and the properties of the synapse. In particular, synapses have different baseline probabilities for releasing vesicles. Synapses with a high release probability will be more likely to show poststimulus depression, whereas those with low release probability are less likely to deplete their vesicle store and thus can be facilitated more easily. Sometimes mixed responses can occur. For example, during a tetanic stimulus train a synapse may show a depressed response, but after the train the synapse can show posttetanic facilitation once the vesicles are recycled.
|
| Presynaptic Receptors Can Modulate Transmitter Release
|
| Just as the postsynaptic membrane contains receptors for neurotransmitters, so does the presynaptic membrane. When these presynaptic receptors bind neurotransmitter, they cause events that can modulate subsequent release of transmitter by the terminal. There are several sources of transmitter that binds to presynaptic receptors: it can be the transmitter released by the terminal itself (i.e., self-modulation, in which case the receptors are referred to as autoreceptors), it can be released by another presynaptic terminal that synapses onto the terminal (a serial synapse), or it can be a nonsynaptically acting neurotransmitter (see the section Neurotransmitters).
|
| Presynaptic receptors can be either ionotropic or metabotropic. In the latter case, recall that their action will be relatively slow in onset and long in duration and the effect will depend on the specific second messenger cascades that are activated. Such cascades can ultimately regulate presynaptic voltage-gated Ca++ and K+ channels and other presynaptic proteins and thereby alter the probability of vesicle release.
|
| In contrast, activation of presynaptic ionotropic receptors will directly alter the electrical properties of the presynaptic terminal and cause rapid transient (millisecond time scale) changes in the probability of vesicle release (although they too can have much longer lasting effects). Binding of an ionotropic receptor will open channels in the presynaptic terminal and thereby alter the amount of transmitter released by an action potential.
|
| Figure 6-10 Presynaptic inhibition. Active regeneration of action potentials in axon 2 ends at the last node. The action potential is then passively conducted into the terminal. Axon 1 makes an axoaxonic synapse with axon 2. Activation of this synapse reduces conduction of the action potential in axon 2 to the active zone of its synaptic terminal by mechanisms described in the text. This reduces the opening of voltage-gated Ca++ channels and therefore release of neurotransmitter. |
| Presynaptic inhibition refers to occasions when binding of presynaptic receptors leads to a decrease in release of transmitter, and it can be the result of one
or more mechanisms (Fig. 6-10). First, opening of channels decreases membrane resistance and creates a current shunt. The shunt acts to divert the current associated with the action potential from the active zone membrane and thereby lessens the depolarization of the active zone, which results in less activation of Ca++ channels, less Ca++ entry, and less release of transmitter. A second mechanism is the change in membrane potential caused by the opening of presynaptic ionotropic channels. If a small depolarization is the result, there will be inactivation of Na+ channels and thereby lessening of the action potential-associated current and transmitter release. Presynaptic γ-aminobutyric acid A receptors (GABAA) occur in the spinal cord and mediate presynaptic inhibition by these mechanisms. They control Cl- channels. Generally, opening of Cl- channels generates a hyperpolarization. However, in the presynaptic terminal, the [Cl-] gradient is such that Cl- flows out of the cell and generates a small depolarization. This depolarization is small enough that it does not cause significant opening of voltage-gated Ca++ channels; otherwise, it would increase release of transmitter (presynaptic facilitation). In fact, there are other receptors that control cation channels and create large depolarizations, thereby increasing the release of transmitter. In addition, presynaptic nicotinic acetylcholine receptors control a cation channel that is permeable to Ca++. By allowing additional entry of Ca++, these receptors increase the release of transmitter from the terminal.
|
| Long-Term Changes in Synaptic Strength
|
| Repetitive stimulation of certain synapses in the brain can also produce more persistent changes in the efficacy of transmission at these synapses, a process called long-term potentiation or long-term depression. Such changes can persist for days to weeks and are believed to be involved in the storage of memories.
|
| page 94 |  | | page 95 |
| The increased synaptic efficacy that occurs in long-term potentiation probably involves both presynaptic (greater transmitter release) and postsynaptic (greater
sensitivity to transmitter) changes, in contrast to the short-term changes that involve changes only in presynaptic function. Entry of calcium into the postsynaptic region is an early step required for initiating the changes that result in long-term enhancement of the response of the postsynaptic cell to neurotransmitter. Entry of calcium occurs through NMDA and some AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) receptors (classes of glutamate receptors; see the section Receptors). Entry of Ca++ is believed to activate Ca++-calmodulin kinase II, a multifunctional protein kinase that is present in very high concentrations in postsynaptic densities. In the presence of high [Ca++], this kinase can phosphorylate itself and thereby become active. Calcium-calmodulin kinase II is believed to phosphorylate proteins that are essential for the induction of long-term potentiation. Long-term potentiation may also have an anatomic component. After appropriate stimulation of a presynaptic pathway, the number of dendritic spines and the number of synapses on the dendrites of postsynaptic neurons may increase rapidly. Changes in the presynaptic nerve terminal may also contribute to long-term potentiation. The postsynaptic neuron may release a signal (nitric oxide has been suggested) that enhances release of transmitter by the presynaptic nerve terminal.
|
| Neurotransmitters are the substances that mediate chemical signaling between neurons. For a substance to be considered a neurotransmitter, it must meet several generally recognized criteria. First, the substance must be demonstrated to be present in the presynaptic terminal and the cell must be able to synthesize the substance. It should be released on depolarization of the terminal. Finally, there should be specific receptors for it on the postsynaptic membrane. This last criterion is certainly true for substances that act as synaptic transmitters, but if we want to be inclusive and include substances that act over widespread territories rather than just at a single synapse, the last criterion needs to be relaxed to include situations in which receptors are located at sites outside the synapse. Neurotransmission has been suggested as a general term to describe both synaptic and nonsynaptic signaling between cells.
|
| More than 100 substances have been identified as potential neurotransmitters because they have met some (hence the "potential" qualifier) or all of these criteria. These substances can be subdivided into three major categories: small-molecule transmitters, peptides, and gaseous transmitters. The small-molecule neurotransmitters may be further subdivided into acetylcholine, amino acids, biogenic amines, and purines. The first three groups on the small-molecule transmitter list contain what are considered the classic neurotransmitters. Remaining transmitters are substances that are more recent additions to the list of neurotransmitters, although many of them have been known as biologically important molecules in other contexts for a long time.
|
| Small-Molecule Neurotransmitters
|
| In the peripheral nervous system, acetylcholine is the transmitter at neuromuscular junctions, at sympathetic and parasympathetic ganglia, and of the postganglionic fibers from all parasympathetic ganglia and a few sympathetic ganglia. It is also a transmitter within the CNS, most prominently of neurons in some brainstem nuclei, in several parts of the basal forebrain (septal nuclei and nucleus basalis) and basal ganglia, and in the spinal cord (e.g., motor neuron axon collaterals). Cholinergic neurons from the basal forebrain areas project diffusely throughout the neocortex and to the hippocampus and amygdala, and they have been implicated in memory functions. Indeed, degeneration of these cells occurs in Alzheimer's disease, a form of dementia in which memory function is gradually and progressively lost.
|
| A number of drugs, known as anticholinesterases, interfere with acetylcholinesterase and thereby prolong the action of acetylcholine at its synapses. Such drugs include insecticides and chemical warfare agents, as well as some therapeutic drugs, such as those used to treat myasthenia gravis. Myasthenia gravis is an autoimmune disease in which antibodies bind to acetylcholine receptors at the neuromuscular junction, thereby disrupting their functionality and causing them to be more rapidly degraded. This reduction in receptors leads to severe weakness and ultimately paralysis. The weakness is characterized by rapid tiring of the muscle with repeated use. Rapid tiring occurs because the number of presynaptic vesicles available for release drops during the high-frequency train of motor neuron action potentials that generates such contractions. Normally, because of the high safety factor of the neuromuscular junction, smaller but still suprathreshold EPPs would still be generated and maintain muscle contraction during repeated use, but in people with myasthenia gravis, the safety factor is so reduced by the loss of acetylcholine receptors that the decrease in release of acetylcholine with repeated activity leads to EPPs that fail to trigger spikes and thus muscular contraction fails. Standard treatments include anticholinesterases, which allow a greater concentration of acetylcholine to partially overcome the deficit caused by the reduced number of functional postsynaptic receptors, and immunosuppressive therapies and plasma exchange, which reduce levels of autoantibodies against the acetylcholine receptor. These therapies are all relatively nonspecific and can therefore have many side effects. Potential future therapies are being developed and include inducing tolerance to the acetylcholine receptor and selective destruction of the B cells that make antibodies against the receptor. |
 |
| page 95 |  | | page 96 |
| Acetylcholine is synthesized from acetyl coenzyme A and choline by the enzyme choline acetyltransferase, which is located in the cytoplasm of cholinergic
presynaptic terminals. After synthesis, acetylcholine is concentrated in vesicles. After release, the action of acetylcholine is terminated by the enzyme acetylcholinesterase, which is highly concentrated in the synaptic cleft. Acetylcholinesterase hydrolyzes acetylcholine into acetate and choline. The choline is then taken up by an Na+ symporter in the presynaptic membrane for the resynthesis of acetylcholine. The extracellular enzymatic degradation of acetylcholine is unusual for a neurotransmitter inasmuch as the synaptic action of other classic neurotransmitters is terminated via reuptake by a series of specialized transporter proteins.
|
| A variety of amino acids function as neurotransmitters. The three most important are glutamate, glycine, and GABA.
|
| Glutamate is the neurotransmitter at the overwhelming majority of excitatory synapses throughout the CNS. Despite its ubiquity, it was initially difficult to identify specific neurons as glutamatergic because glutamate is present in all cells; it has a key role in multiple metabolic pathways, and it is a precursor to GABA, the major inhibitory neurotransmitter. Nevertheless, experimental results have now clearly established glutamate as the major excitatory CNS neurotransmitter. When applied to cells, it causes depolarization and is released from neurons, and specific receptors and transporters for it have been identified.
|
| In addition to being the main excitatory neurotransmitter, glutamate is a potent neurotoxin at high concentrations. Thus, strict limitation of glutamate's activity after its release from the presynaptic terminal is necessary, not only to allow normal synaptic transmission but also to prevent cell death. This task is accomplished by specialized membrane transporter proteins.
|
| GABA and glycine act as inhibitory neurotransmitters. GABA is the major inhibitory transmitter throughout the nervous system. GABA is produced from glutamate by a specific enzyme (glutamic acid decarboxylase) that is present only in neurons that use GABA as a transmitter. Thus, experimentally, it is possible to identify cells as inhibitory GABA-ergic neurons by using antibodies to this enzyme to mark them (immunolabeling). Many local interneurons are GABA-ergic. In addition, several brain regions contain large numbers of GABA-ergic projection neurons. The most notable are the spiny neurons of the striatum and the Purkinje cells of the cerebellar cortex. The inhibitory nature of Purkinje cells was especially surprising because they represent the entire output of the cerebellar cortex, and thus cerebellar cortical activity basically functions to suppress the activity of its downstream targets (cerebellar and vestibular nuclei).
|
| Glycine functions as an inhibitory neurotransmitter in a much more restricted territory. Glycinergic synapses are predominantly found in the spinal cord, where they represent approximately half of the inhibitory synapses. They are likewise present in the lower brainstem, cerebellum, and retina in significant numbers. Interestingly, glycine also has another synaptic function. At excitatory NMDA-type glutamate receptors, glycine must also be bound for the ion channel to open. Thus, it acts as a cotransmitter at these synapses. It was generally thought that under physiological conditions the extracellular glycine concentration was high enough that the glycine binding sites of the NMDA channel were always saturated, but recent results suggest that this may not always be true, which implies that fluctuations in glycine levels may also be an important modulator of NMDA-mediated synaptic transmission.
|
| After GABA and glycine are released from the presynaptic terminal, they are taken back up into the nerve terminal and neighboring glia by high-affinity Na+-Cl--coupled membrane transporters. These Na+-Cl- transporters are part of a superfamily of transporters that also includes those for the biogenic amine neurotransmitters, but it is distinct from those for glutamate. Transport of the neurotransmitter into the cell is accomplished by symport with two Na+ and one Cl- ion. There are four GABA transporters (GAT1 to GAT4), which are found on neurons and glia, the exact distribution varying by subtype. There are two main glycine transporters, GlyT1 and GlyT2. GlyT1 is found predominantly on astrocytes and is present throughout the CNS. In contrast, GlyT2 is located on glycinergic nerve terminals and is largely restricted to the spinal cord, brainstem, and cerebellum.
|
| Many of the neurotransmitters in this category may be familiar because they have roles outside the nervous system, often as hormones. Among the amines known to act as neurotransmitters are dopamine, norepinephrine (noradrenaline), epinephrine (adrenaline), serotonin (5-hydroxytryptamine [5-HT]), and histamine. Dopamine, norepinephrine, and epinephrine are catecholamines, and they share a common biosynthetic pathway that starts with the amino acid tyrosine. Tyrosine is converted to l-dopa by the enzyme tyrosine hydroxylase. l-Dopa is then converted to dopamine by dopa-decarboxylase. In dopaminergic neurons, the pathway stops here. In noradrenergic neurons, another enzyme, dopamine β-hydroxylase, converts dopamine to norepinephrine. Epinephrine is obtained by adding a methyl group to norepinephrine via phenylethanolamine-N-methyl transferase. In serotoninergic neurons, serotonin is synthesized from the essential amino acid tryptophan. Tryptophan is first converted to 5-hydroxytryptophan by tryptophan 5-hydroxylase, which is then converted to serotonin by aromatic l-amino acid decarboxylase. Finally, in histaminergic neurons the conversion of histidine to histamine is catalyzed by histidine decarboxylase.
|
| Removal of synaptically released biogenic amines is generally accomplished by reuptake into glia and neurons via transporters belonging to the Na+-Cl--dependent transporter family. The catecholamines are then degraded by two enzymes, monoamine oxidase and catechol O-methyltransferase.
|
| page 96 |  | | page 97 |
| At least five transporters (called EAAT1 to EAAT5, where EAAT stands for excitatory amino acid transporter) that carry glutamate across the plasma membrane have been identified. They are all part of the Na+-K+-dependent family of transporters. Inward movement of each glutamate molecule is driven by the cotransport of three Na+ ions and one H+ ion and the countertransport of one K+ ion out of the cell (Fig. 6-11B). In addition, the transporter has Cl- conductance, although passage of Cl- ions is not stoichiometrically linked to glutamate transport. Glutamate transporters are found on both neurons and glia. However, the transporters differ in their regional and cellular distribution and in their pharmacological and biophysical properties. For example, EAAT2 is found on glia and is generally responsible for more than 90% of glutamate uptake from the extracellular space. The glutamate taken up into glial cells by EAAT2 is eventually returned to the presynaptic terminal by the glutamate-glutamine cycle (Fig. 6-11). Inside glial cells glutamate is converted to glutamine. Glutamine is then transported out of the glial cell and back into the presynaptic terminal, where it is subsequently converted back to glutamate. Glutamate inside the presynaptic terminal is packaged into synaptic vesicles by a second set of glutamate transporters known as vGLUTs (vesicular glutamate transporters), which are present in the membrane of glutamatergic vesicles. Transport of glutamate into synaptic vesicles by vGLUT is driven by the countertransport of H+ ions, the electrochemical gradient for which having been established by an H+-ATPase in the vesicle membrane. |
 |
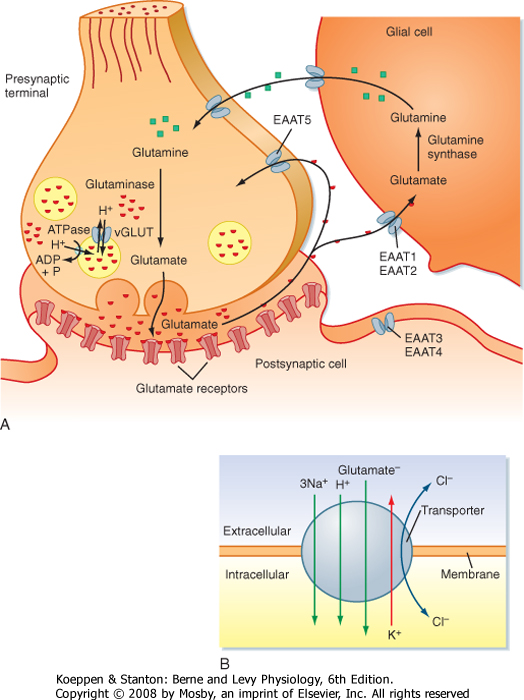
|
| Figure 6-11 Glutamate transport cycle. (A) A schematic shows the fate of glutamate released from a presynaptic terminal. Distinct glutamate transporters exist on the presynaptic and postsynaptic cell membranes for reuptake. In addition, glial cells take up glutamate and convert it to glutamine. The glutamine is then released and taken into the presynaptic terminal, where it is converted back to glutamate before being repackaged into synaptic vesicles. (B) Schematic of transporter showing direction of ion flow associated with the movement of glutamate across the membrane. |
| page 97 |  | | page 98 |
| Within the CNS, nerve cells that use biogenic amines as neurotransmitters are primarily found within one of
a few brainstem nuclei, most of which project rather diffusely throughout large areas of the brain. Noradrenergic neurons are primarily found in the locus ceruleus and nucleus subceruleus, which are located near each other in the tegmentum of the rostral pons. The neurons of the locus ceruleus project throughout the entire brain. Targets of the nucleus subceruleus are more limited, but still widespread and include the pons, medulla, and spinal cord. (Norepinephrine is also important in the peripheral nervous system because it is used by postganglionic sympathetic cells.) Serotoninergic fibers arise from a series of nuclei located at the midline of the brainstem, known as the raphe nuclei. Similar to the noradrenergic fibers, serotoninergic fibers are distributed throughout most of the brain and spinal cord. Dopaminergic fibers arise from two main brainstem regions: the substantia nigra pars compacta, which projects to the striatum, and the ventral tegmental area, which projects more widely to the neocortex and subcortical areas. Histaminergic neurons are located within the tuberomammillary nucleus of the hypothalamus but project diffusely throughout the CNS. Finally, adrenergic neurons are relatively few in number when compared with the other biogenic amine transmitters, but they too have cell bodies localized to small cell groups in the rostral medulla. The largest group, termed C1, has projections to the locus ceruleus and down to the thoracic and lumbar levels of the spinal cord, where they terminate in the autonomic nuclei of the intermediolateral and intermediomedial cell columns. Thus, these neurons are important for autonomic functions, particularly vasomotor ones, such as control of arterial pressure.
|
| The diffuse nature of the projection pattern of most of the amine systems is mirrored in their proposed functions. Activity in the different aminergic systems is believed to be important in setting global brain states. For example, these systems are involved in setting the level of arousal (sleep, waking), attention, and mood. Their involvement in pathways connected with the hypothalamus and other autonomic centers also indicates that they have important homeostatic functions. The role of dopamine in balancing the flow of activity through the basal ganglia pathways and how its loss leads to the motor symptoms observed in Parkinson's disease are described in Chapter 9.
|
| Hyperactivity of dopaminergic synapses may be involved in some forms of psychosis. Chlorpromazine and related antipsychotic drugs inhibit dopamine receptors on postsynaptic membranes and thus diminish the effects of dopamine released from presynaptic nerve terminals. Overdoses of such antipsychotic drugs can produce a temporary parkinsonian-like state. |
 |
| ATP has the potential to act as a transmitter or cotransmitter at synapses in the peripheral and central
nervous systems. ATP is found in all synaptic vesicles and thus is coreleased during synaptic transmission. ATP has its own receptors, which like standard neurotransmitters, are coupled to ion channels, but it can also modify the action of other neurotransmitters with which it is coreleased, including norepinephrine, serotonin, glutamate, dopamine, and GABA. Glial cells may also release ATP after certain types of stimulation. Once released, ATP is broken down by ATPases and 5-nucleotidase to adenosine, which can be taken up again by the presynaptic terminal.
|
| Peptide neurotransmitters consist of chains of between 3 and about 40 amino acids. Studies of neuropeptides focused on the hypothalamus for many years. However, it is now clear that neuropeptides are released by neurons and act on receptors throughout the CNS and thus are a fundamental mechanism of neurotransmission throughout the CNS. To date, more than 100 neuropeptides have been identified. They can be classified into several functional groups, as shown in Table 6-2, which lists some of the known neuropeptides. It is now clear that many neurons that release classic neurotransmitters also release neuropeptides. As detailed later, understanding the interaction between coexisting classic and peptide transmitters has become an important area of research. In addition to being co-released with another transmitter, neuropeptides can also function as the sole or primary neurotransmitter at a synapse.
|
| In some ways neuropeptides are like the classic neurotransmitters: they are packaged into synaptic vesicles, their release is dependent on Ca++, and they bind to specific receptors on target neurons. However, there are also significant differences, ones that have led to alternative names for the intercellular communication mediated by neuropeptides, such as nonsynaptic, parasynaptic, and volume transmission. Table 6-1 summarizes some of these differences between classic and peptide neurotransmitters.
|
| Unlike classic neurotransmitters, which are synthesized at the presynaptic terminal, neuropeptides are synthesized at the cell body and then transported to the terminal (Fig. 6-2). Neuropeptides are packaged into large electron-dense vesicles that are scattered throughout the presynaptic terminal rather than in small electron-lucent vesicles docked at the active zone, where small-molecule transmitters are stored. (In neurons that make multiple neuropeptides, the various peptides are costored in the same vesicles.) Neuropeptide receptors are not confined to the synaptic region, and in general, peptide action is not limited by reuptake mechanisms.
|
| Each of these differences has functional implications. For example, the separate storage of peptide and nonpeptide transmitters immediately raises the question of whether the two transmitters are co-released or differentially released in response to particular stimulation patterns.
|
| page 98 |  | | page 99 |
|
Table 6-2.
Some Neuroactive Peptides |
| Hypothalamic Hormones |
| Corticotropin-releasing hormone (CRH) |
| Growth hormone-releasing hormone (GHRH) |
| Luteinizing hormone-releasing hormone (LHRH) |
| Oxytocin |
| Somatostatin |
| Thyrotropin-releasing hormone (TRH) |
| Vasopressin |
| NPY-Related Peptides |
| Neuropeptide Y |
| Opioid Peptides |
| Dynorphin |
| Methionine enkephalin |
| Leucine enkephalin |
| Tachykinins |
| Neurokinin α |
| Neurokinin β |
| Neuropeptide K |
| Substance P |
| VIP-Glucagon Family |
| Glucagon-like peptide 1 |
| Peptide histidine-leucine |
| Pituitary adenylyl cyclase-activating peptide (PACAP) |
| Vasoactive intestinal polypeptide (VIP) |
| Others |
| Adrenocorticotropic hormone (ACTH) |
| Brain natriuretic peptide |
| Cholecystokinin (CCK) |
| Galanin |
| Hypocretins/orexins |
| Neurotensin |
| Motilin |
| Insulin |
| α-Melanocyte-stimulating hormone (α-MSH) |
| Neurotensin |
| Prolactin-releasing peptide |
| Secretoneurin |
| Urocortin |
| In fact, differential release of peptide and classic transmitters from the same cell has been demonstrated
for several types of neurons and is probably a result of the differences in vesicle storage described earlier. Because of their proximity to the active zones, nonpeptide vesicles can be released rapidly (<1 msec) in response to single action potentials as a result of localized influx of Ca++. Thus, low-frequency stimulation of the cell causes just the release of nonpeptide transmitter. In contrast, with higher-frequency stimulation of the presynaptic neuron, there is a more global increase in [Ca++] throughout the nerve terminal that leads to release of neuropeptide, as well as neurotransmitter.
|
| When neuropeptides are coreleased with other transmitters, they may act synergistically or antagonistically. For example, in the spinal cord, tachykinins and calcitonin gene-related peptide (CGRP) act synergistically with glutamate and with substance P to enhance the action of serotonin. Conversely, tachykinins and CGRP antagonize norepinephrine's action at other synapses. The interactions, however, are not simply a one-to-one synergism or antagonism at a particular synapse because of the differing temporal and spatial profiles of the action of peptides versus classic transmitters. In particular, the slower release and lack of rapid reuptake mean that neuropeptides can act for long durations, diffuse over a region of brain tissue, and affect all cells in that region (that have the appropriate receptors) rather than just acting at the specific synapse at which it was released. In fact, studies have shown that there is often a spatial mismatch between the presynaptic terminals that contain a particular neuropeptide and the sites of the receptors for that peptide. In sum, peptides released from a particular synapse probably affect the local neuronal population as a whole, whereas the coreleased classic transmitters act in more of a point-to-point manner.
|
| Opiates are drugs derived from the juice of the opium poppy. Compounds that are not derived from the opium poppy but that exert direct effects by binding to opiate receptors are called opioids and form a clinically and functionally important class of neuropeptides. Operationally, opioids are defined as compounds whose effects are stereospecifically antagonized by a morphine derivative called naloxone.
|
| The three major classes of endogenous opioid peptides in mammals are enkephalins, endorphins, and dynorphins. Enkephalins are the simplest opioids; they are pentapeptides. Dynorphin and the endorphins are somewhat longer peptides that contain one or the other of the enkephalin sequences at their N-terminal ends.
|
| Opioid peptides are widely distributed in neurons of the CNS and intrinsic neurons of the gastrointestinal tract. The endorphins are discretely localized in particular structures of the CNS, whereas the enkephalins and dynorphins are more widely distributed. Opioids inhibit neurons in the brain involved in the perception of pain. Indeed, opioid peptides are among the most potent analgesic (pain-relieving) compounds known, and opiates are used therapeutically as powerful analgesics. They exert their analgesic effect by binding to specific opiate receptors.
|
| Substance P is a peptide consisting of 11 amino acids. It is present in specific neurons in the brain, in primary sensory neurons, and in plexus neurons in the wall of the gastrointestinal tract. The wall of the gastrointestinal tract is richly innervated with neurons that form networks or plexuses (see also Chapter 32). The intrinsic plexuses of the gastrointestinal tract exert primary control over its motor and secretory activities. These enteric neurons contain many of the neuropeptides, including substance P, that are found in the brain and spinal column. Substance P is involved in pain transmission and has a powerful effect on smooth muscle.
|
| page 99 |  | | page 100 |
| Substance P is probably the transmitter used at synapses made by primary sensory neurons (their cell
bodies are in the dorsal root ganglia) with spinal interneurons in the dorsal horn of the spinal column, and thus it is an example of a peptide acting as a primary transmitter at a synapse. Enkephalins act to decrease the release of substance P at these synapses and thereby inhibit the pathway for pain sensation at the first synapse in the pathway.
|
| This is the newest category of neurotransmitter to be defined and stretches the usual definition of synaptic transmission even further than neuropeptides do. Gas neurotransmitters are neither packaged into synaptic vesicles nor released by exocytosis. Instead, gas neurotransmitters are highly permeant and simply diffuse from synaptic terminals to neighboring cells after synthesis, their synthesis being triggered by depolarization of the nerve terminal (the influx of Ca++ activates synthetic enzymes). Moreover, there are no specific reuptake mechanisms, nor do they undergo enzymatic destruction, so their action appears to be ended by diffusion or binding to superoxide anions or various scavenger proteins. Both nitric oxide (NO) and carbon monoxide (CO) are examples of gaseous neurotransmitters. NO is a transmitter at synapses between inhibitory motor neurons of the enteric nervous system and gastrointestinal smooth muscle cells (see Chapter 32). NO also functions as a neurotransmitter in the CNS. The enzyme NO synthase catalyzes the production of NO as a product of the oxidation of arginine to citrulline. This enzyme is stimulated by an increase in cytosolic [Ca++].
|
| In addition to serving as a neurotransmitter, NO functions as a cellular signal transduction molecule both in neurons and in nonneuronal cells (such as vascular smooth muscle, see Chapter 14). One way that NO functions as a signal transduction molecule is by regulating guanylyl cyclase, the enzyme that produces cGMP from GTP. NO binds to a heme group in soluble guanylyl cyclase and potently stimulates the enzyme. Stimulation of this enzyme leads to an elevation in cGMP in the target cell. The cGMP can then influence multiple cellular processes.
|
| NEUROTRANSMITTER RECEPTORS
|
| The multitude of neurotransmitters used in the nervous system provides it with a specific and flexible interneuronal communications system. These characteristics are even further enhanced by the variety of receptors for each neurotransmitter. Receptors for a particular neurotransmitter were traditionally distinguished primarily by pharmacological differences in their sensitivity to particular agonists and antagonists. For example, acetylcholine receptors were split into muscarinic and nicotinic classes, depending on whether they bind nicotine or muscarine. Similarly, glutamate receptors were split into three main groups according to their sensitivity to the agonists NMDA, kainic acid, or AMPA. Though useful, this classification scheme has several limitations: some receptors fail to be activated by agonists, and it fails to disclose all the various receptor subtypes for a particular transmitter. Over the past 15 years or so, molecular biological approaches have been used to identify and sequence the receptor genes for many of the known neurotransmitters. It is thought that we now have a relatively complete catalog of the genes for these receptors. What this work has revealed is that there is a tremendous diversity of actual and potential receptor subtypes that are or could be used by the nervous system. Moreover, knowledge of the gene sequences has enabled an understanding of the relationship of different receptor proteins to each other and to other important proteins. This knowledge, combined with the results of biochemical, crystallographic, and other types of studies, has led to a much deeper understanding of the structural and functional workings of receptor proteins. In particular, various receptors can be grouped into families based on gene sequences, and members of each family share various structural and functional features.
|
| Neurotransmitter receptors are members of one of two large groups or families of proteins: ligand-gated ion channels, also known as ionotropic receptors, and G protein-coupled receptors, also referred to as metabotropic receptors (Fig. 6-12, A and B). Almost all classic neurotransmitters and neuropeptides have at least one metabotropic-type receptor. Many of the classic neurotransmitters also have at least one ionotropic receptor. Ionotropic receptors are protein complexes that both have an extracellular binding site for the transmitter and form an ion channel (pore) through the cell membrane. The receptor is made up of several protein subunits, usually three to five, each of which typically has a series of membrane-spanning domains, some of which contribute to the wall of the ion channel. Binding of the neurotransmitter alters (usually increases) the probability of the ion channel being in the open state and thus typically results in postsynaptic events that are rapid in both onset and decay, with a duration of several milliseconds. Ionotropic receptors underlie fast synaptic EPSPs and IPSPs, as described earlier.
|
| The ionotropic receptors can be divided into several superfamilies. Members of the cys-loop superfamily have peptide subunits that have an N-terminal extracellular domain that contains a loop delimited by cysteine residues. This family includes the ionotropic receptors for acetylcholine, serotonin, GABA, and glycine. In addition to their family-defining cysteine loop, these receptors share the following common features: they are pentamers, with each peptide subunit having four transmembrane domains; the neurotransmitter binds to the N-terminal domain; and the second transmembrane domains are thought to form the wall of the ion pore. |
| Ionotropic glutamate and ATP receptors form two other ionotropic receptor superfamilies; the details for each are given in the later corresponding sections. Transient receptor potential channels, which are important for transduction of pain and thermal sensations, form yet another family (see Chapter 7). |
| page 100 |  | | page 101 |
| Figure 6-12 Neurotransmitter receptors. The basic structure and mechanism of action are shown for ligand-gated ion channels (ionotropic receptors) (A) and G protein-coupled (metabotropic) receptors (B). Detailed structures of cys-loop and glutamate ionotropic receptors are shown in C and D, respectively. Cys-loop receptors include ionotropic receptors for GABA, glycine, serotonin, and acetylcholine. Note the differing membrane topologies of the individual subunits of these two classes of receptors: four transmembrane domains for cys-loop receptors and three plus a pore loop for glutamate receptors. Pore loops form the internal wall of the glutamate channel, whereas transmembrane domain 2 forms the internal wall of cys-loop receptors. (A and B Purves D, Augustine GJ, Fitzpatrick D, Neuroscience, 2nd ed. Sunderland, MA, Sinaver Associates, 2001.) |
| page 101 |  | | page 102 |
| Metabotropic receptors are not ion channels. Instead, they are protein monomers that have an extracellular binding site for a particular transmitter and an intracellular site for binding a G protein. Binding of the receptor leads to activation of a G protein, which is the first step in a signal transduction cascade that alters the function of an ion channel in the postsynaptic membrane. In contrast to ionotropic receptors, metabotropic receptors mediate postsynaptic phenomena that have a slow onset and that may persist from hundreds of milliseconds to minutes. Because of the various biochemical cascades that they initiate, they have great potential to cause changes in the neuron beyond just generating a postsynaptic potential.
|
| Acetylcholine receptors were originally classified on a pharmacological basis (being sensitive to nicotine or muscarine) into two major groups. This grouping corresponds to groupings based on structural and molecular biological studies. Nicotinic receptors are members of the ionotropic cys-loop family, and muscarinic receptors are part of the metabotropic family of receptor proteins.
|
| The nicotinic receptors mediate synaptic transmission at the neuromuscular junction, as described earlier; however, nicotinic receptors are also present within the CNS. The nicotinic receptor contains a relatively nonselective cationic channel, so binding of acetylcholine produces an EPSP. Being members of the cys-loop family, acetylcholine receptors are pentamers constructed from a series of subunit types called α, β, γ, δ, Ε, some of which contain multiple members. At the neuromuscular junction the channel is constructed from 2α, β, δ, Ε, whereas in the CNS, the composition is typically 3α, 2β. Furthermore, the junctional receptors all use the α1 subunit, whereas centrally located receptors use one of the α subunits between α2 and α10. As noted, the differing subunits result in receptors with differing pharmacological sensitivities and channel kinetics and selectivity.
|
| There are five known muscarinic subtypes of acetylcholine receptors (M1 to M5). All are metabotropic receptors; however, they are coupled to different G proteins and can thus have distinct effects on the cell. M1, M3, and M5 are coupled to pertussis toxin-insensitive G proteins, whereas M2 and M4 are coupled to pertussis toxin-sensitive G proteins. Each set of G proteins is coupled to different enzymes and second messenger pathways (see Chapter 3 for details of these pathways).
|
| Inhibitory Amino Acid Receptors: GABA and Glycine
|
| As noted, the most common inhibitory synapses in the CNS use either glycine or GABA as their transmitter. Glycine-mediated inhibitory synapses predominate in the spinal cord, whereas GABAergic synapses make up the majority of inhibitory synapses in the brain.
|
| Both glycine and GABA (GABAA and GABAC) have ionotropic receptors that are members of the cys-loop family, thus sharing a number of characteristics, as already described. In addition, each of these receptors has a Cl- channel, which opens while the receptor portion is bound. Therefore, the probability of these channels opening and the average time that a channel stays open are controlled by the concentration of the neurotransmitter for which the receptor is specific.
|
| Glycine receptors are pentamers and may be heteromers of α and β subunits (3 : 2 ratio) or homomers. Interestingly, the molecular composition appears to be related to its cellular location, with heteromers located postsynaptically and homomers located extrasynaptically. The β subunit seems to bind to an intracellular scaffold protein called gephyrin that appears to help localize receptors to the postsynaptic site. The α subunit contains the glycine binding site, and there are four genes coding for distinct α subunits (and splice variants of each). Each variant results in a receptor having distinct conductance, kinetics, agonist and antagonist affinity, and modulatory sites. Intriguingly, subunit variants are differentially expressed during development and in different brain regions.
|
| GABA has two separate ionotropic receptors (GABAA and GABAC) coded for by distinct sets of genes. Like glycine receptors, both control a Cl- channel. GABAA receptors are heteromers generated from seven classes of subunits, three of which have multiple members. The most common configuration is α1, β2, γ2 in a 2 : 2 : 1 stoichiometry, which may account for 80% of the receptors; however, many other heteromers are found in the brain. As with glycine, different subunits confer distinct properties on the receptor. For example, GABAA receptors are the targets of two major classes of drugs: benzodiazepines and barbiturates. Benzodiazepines (e.g., diazepam) are widely used antianxiety and relaxant drugs. Barbiturates are used as sedatives and anticonvulsants. Both classes of drugs bind to distinct sites on the α subunits of GABAA receptors and enhance opening of the receptors' Cl- channels in response to GABA. The sedative and anticonvulsant actions of benzodiazepines appear to be mediated by receptors with the α1 subunit, whereas the anxiolytic effects reflect binding to receptors with the α2 subunit. GABAC receptors are structurally similar to GABAA receptors but have a distinct pharmacological profile (e.g., they are not affected by benzodiazepines) and are coded for by a separate set of genes (ρ1, ρ2, and ρ3).
|
| The GABAB receptor is a metabotropic receptor. Binding of GABA to this receptor activates a heterotrimeric GTP-binding protein (G protein, see Chapter 3), which leads to activation of K+ channels and hence hyperpolarization of the postsynaptic cell, as well as inhibition of Ca++ channels (when located presynaptically) and thus a reduction in release of transmitter.
|
| Excitatory Amino Acid Receptors: Glutamate
|
| page 102 |  | | page 103 |
| Glutamate has both ionotropic and metabotropic receptors. Based on pharmacological properties and subunit composition, several distinct ionotropic receptor subtypes are recognized: AMPA, kainate, and NMDA. Overall, there are 18 known genes that code for glutamate subunits for the ionotropic glutamate receptors. However, the genes are divided into several
families (AMPA, kainate, NMDA, and δ) that essentially correspond to the pharmacological subtypes of receptors. Each glutamate receptor is a tetramer. Thus, there is a certain correspondence between the genes and the receptor types that are formed. For example, AMPA receptors are formed from GluR1 to GluR4 subunits, kainate receptors require either KA1 or KA2 and GluR5 to GluR7 subunits, and NMDA receptors all have NR1 subunits plus some combination of NR2 and NR3 subunits. As was mentioned for the other receptors, the various receptor properties vary with subunit composition. Ionotropic glutamate receptors are excitatory and contain a cationic-selective channel. Thus, all the channels are permeable to Na+ and K+, but only a subset allow Ca++ to pass.
|
| AMPA and kainate receptors behave as classic ligand-gated channels, as already discussed; on binding of glutamate to the receptor, the channel opens and allows current to flow, thereby generating an EPSP. NMDA channels are different. First, they require binding of both glutamate and glycine to open. Second, they display voltage sensitivity as a result of Mg++ blockade of the channel. That is, at resting (or more negative) membrane potentials, a Mg++ ion blocks the entrance to the channel so that even when glutamate and glycine are bound, no current flows through the channel. However, if the cell is depolarized (either experimentally by injection of current through a recording electrode or by other EPSPs), the Mg++ block is relieved and current can flow through the channel. A further interesting feature of NMDA channels is that they are generally permeable to Ca++, which can act as a second messenger. The combination of voltage sensitivity and Ca++ permeability of the NMDA channels has led to hypotheses concerning their role in learning and memory-related functions (see Chapter 10).
|
| Eight genes coding for metabotropic glutamate receptors have been identified and classified into three groups. Group I receptors are found postsynaptically, whereas groups II and III are found presynaptically. These receptors generate slow EPSPs, but probably at least as importantly, they trigger second messenger cascades (see Chapter 3).
|
| Purines have two receptor families: an ionotropic (P2X) and a metabotropic (P2Y) family. There are seven identified P2X subunit types that form channels, and they represent their own superfamily of ligand-gated channels. Each subunit has only two transmembrane domains, with the loop between these two domains located extracellularly and containing the ATP binding site. The receptors are heterotrimers or homotrimers or hexamers. In general, these receptors form a cationic channel that is permeable to Na+, K+, and Ca++. The distribution of subunits in the brain varies significantly, with some subunits having a widespread distribution (P2X2) and others being quite limited (P2X3 is present mostly on cells involved in pain-related pathways).
|
| Metabotropic purine receptors are coded for by 10 genes, but only six are expressed in the human CNS. They have the typical features of G protein-coupled receptors and are known to activate K+ currents and modulate both NMDA and voltage-gated Ca++ currents. An interesting localization distinction between P2X and P2Y receptors is that although both are present on neurons, the latter dominates on astrocytes.
|
| Finally, in addition to the P2X and P2Y receptors, which respond to ATP, there are adenosine receptors that respond to the adenosine that is released after the enzymatic breakdown of ATP. These receptors are located presynaptically and act to inhibit synaptic transmission by inhibiting influx of Ca++.
|
| Biogenic Amine Receptors: Serotonin, Dopamine, Noradrenaline, Adrenaline, Histamine
|
| With the exception of one class of serotonin receptors (5-HT3), which are part of the cys-loop ionotropic family, the receptors for the various biogenic amines are all metabotropic-type receptors. Thus, these neurotransmitters tend to act on relatively long time scales by generating slow synaptic potentials and by initiating second messenger cascades. Agonists and blockers of many of these receptors are important clinical tools for treating various neurological and psychiatric disorders. The role of different dopamine receptors in basal ganglia disorders will be covered in the motor systems (Chapter 9).
|
| As is the case with the biogenic amines, receptors for the various peptides are essentially all of the metabotropic type and are coupled to G proteins that mediate effects via second messenger cascades. It is worth mentioning again that studies consistently show a mismatch between the locations of terminals containing a particular peptide and the receptors for it. Thus, these receptors are often activated by neurotransmitter diffusing through the extracellular space rather than at synapses. This implies that these receptors will experience much lower concentrations of agonist, and indeed, they are more sensitive to their agonists.
|
| Gas Neurotransmitter Receptors
|
| Unlike the other neurotransmitters that were covered, NO and CO do not bind to receptors. One way that they do affect cell activity is to activate enzymes involved in second messenger cascades, such as guanylyl cyclase. In addition, NO has been shown to modify the activity of other proteins, such as NMDA receptors and the Na+,K+-ATPase pump, by nitrosylating them.
|
| page 103 |  | | page 104 |
- Both electrical and chemical synapses are important means of cellular communication in the mammalian nervous system.
- Electrical synapses directly connect the cytosol of two neurons and allow rapid bidirectional current flow between neurons. They act as low-pass filters.
- Gap junctions are the morphological correlate of electrical synapses. Gap junctions contain channels formed by hemichannels called connexons. Connexons are formed by proteins called connexins.
- Standard chemical synaptic transmission involves the release of transmitter from a presynaptic terminal, diffusion of transmitter across a synaptic cleft, and binding of the transmitter to receptors on the apposed postsynaptic membrane.
- Entry of calcium into the presynaptic terminal triggers the release of neurotransmitter. Release of neurotransmitter is quantal, as first demonstrated by the recording of mEPPs at the frog neuromuscular junction.
- Transmitter is packaged into synaptic vesicles in the presynaptic terminal. The vesicles are the quantal elements. That is, the release of transmitter from one vesicle causes an mEPP at the neuromuscular junction or, equivalently, one mPSP at a central synapse.
- Many proteins are involved in priming, docking, and fusion of synaptic vesicles. Synaptotagmin is the Ca++ sensor for triggering vesicle fusion.
- Excitatory and inhibitory synapses increase or decrease, respectively, the probability that the postsynaptic neuron will spike.
- The reversal potential is the membrane potential at which net current flow through a ligand-gated channel reverses. Excitatory synapses generate depolarizing potentials (EPSPs) that have reversal potentials positive to the spike threshold, most often as a result of the opening of nonselective cation channels.
- Inhibitory synapses generate IPSPs that have reversal potentials more negative than the spike threshold, but not necessarily negative to the resting potential. Inhibitory synapses can decrease spike probability by two mechanisms: hyperpolarization of the membrane and a decrease in the input resistance of the neuron leading to a shunt of synaptic currents.
- The efficacy of synaptic transmission depends on the timing and frequency of action potentials in the presynaptic neuron. Facilitation, posttetanic potentiation, and long-term potentiation are examples of increased efficacy of synaptic transmission in response to previous multiple stimulations of a synapse. Long-term depression is an example of reduced efficacy resulting from previous activation of the synapse.
- The nervous system uses hundreds of neurotransmitters. Neurotransmitters can be subdivided into a few broad functional classes: small-molecule transmitters (acetylcholine, amino acids, biogenic amines, and purines), peptides, and gases (CO, NO). The action of a neurotransmitter depends on its postsynaptic receptors. Most nongaseous transmitters have both ionotropic and metabotropic receptors.
- Small-molecule transmitters act locally, mainly across a single synapse, and their duration of action is limited by reuptake and enzymatic degradation. Peptides can diffuse from their presynaptic release site and thus have the potential to affect all cells within a local region. Gaseous transmitters are free to diffuse from their release site.
- Ionotropic receptors contain an ion channel whose state (open versus closed) is gated by the binding of neurotransmitter to the receptor. Metabotropic receptors activate second messengers on binding neurotransmitter.
- Many synapses can release multiple types of transmitters, and which ones they release depends on the activity pattern of the terminal. Coreleased transmitters may function independently or act synergistically or antagonistically.
|
 |
|