| 7 The Somatosensory System
|
| The somatosensory system provides information to the central nervous system (CNS) about the state of the body and its contact with the world. It does so by using a variety of sensory receptors that transduce mechanical (pressure, stretch, and vibrations) and thermal energies into electrical signals. These electrical signals, called generator potentials, occur in the distal ends of axons of first-order somatosensory neurons and trigger action potential trains that reflect information about the characteristics of the stimulus. The cell bodies of these neurons are located in dorsal root (Fig. 7-1, A; and see Fig. 4-8) and cranial nerve ganglia.
|
| Each ganglion cell gives off an axon that after a short distance, divides into a peripheral process and a central process. The peripheral processes of the ganglion cells coalesce to form peripheral nerves. A purely sensory nerve will have only axons from also such ganglion cells; however, mixed nerves, which innervate muscles, will contain both afferent (sensory) fibers and efferent (motor) fibers. At the target organ, the peripheral process of an afferent axon divides repeatedly, with each terminal branch ending as a sensory receptor. In most cases, the free nerve ending by itself forms a functional receptor, but in some, the nerve ending is encapsulated by accessory cells, and the entire structure (axon terminal plus accessory cells) forms the receptor.
|
| The central axonal process of the ganglion cell either enters the spinal cord via a dorsal root or enters the brainstem via a cranial nerve. A central process typically gives rise to numerous branches that may synapse with a variety of cell types, including second-order neurons of the somatosensory pathways. The terminal location of these central branches varies depending on the type of information being transmitted. Some terminate at or near the segmental level of entry, whereas others project to brainstem nuclei.
|
| Second-order neurons that are part of the pathway for the perception of somatosensory information project to specific thalamic nuclei, where the third-order neurons reside. These neurons in turn project to the primary somatosensory cortex (S-I). Within the cortex, somatosensory information is processed in S-I and in numerous higher-order cortical areas. Somatosensory information is also transmitted by other second-order neurons to the cerebellum for use in its motor coordination function.
|
| The organization of the somatosensory system is quite distinct from that of the other senses, which has both experimental and clinical implications. In particular, other sensory systems have their receptors localized to a single organ, where they are present at high density (e.g., the eye for the visual system). In contrast, somatosensory receptors are distributed throughout the body (and head). In addition, the other senses convey their information to the brain via a single nerve bundle (or in one case, via two to three nerves), whereas somatosensory information arrives via spinal dorsal roots and cranial nerves (primarily the trigeminal).
|
| SUBDIVISIONS OF THE SOMATOSENSORY SYSTEM
|
| The somatosensory system receives three broad categories of information based on the distribution of its receptors. Its exteroceptive division is responsible for providing information about contact of the skin with objects in the external world, and a variety of cutaneous mechanoceptive, nociceptive (pain), and thermal receptors are used for this purpose. Understanding this division will be the main focus of this chapter. The proprioceptive component provides information about body and limb position and movement and relies primarily on receptors found in the joints, muscles, and tendons. Because these receptors initiate pathways that in part are intimately involved in the control of movement, they will be discussed in Chapter 9; however, the ascending central pathways that originate with them and that underlie conscious and unconscious proprioceptive functions will be covered later in this chapter. Finally, the enteroceptive division has receptors for monitoring the internal state of the body and includes mechanoreceptors that detect distention of the gut or fullness of the bladder.
|
| page 105 |  | | page 106 |

|
| Figure 7-1 Ascending somatosensory pathways from the body. A, First-, second-, and third-order neurons are shown for the two main pathways conveying cutaneous information from the body to the cerebral cortex: the dorsal column/medial lemniscal and the spinothalamic pathways. Note that the axon of the second-order neuron crosses the midline in both cases, so sensory information from one side of the body is transmitted to the opposite side of the brain, but the levels in the neuraxis at which this takes place are distinct for each pathway. Homologous central pathways for the head originate in the trigeminal nucleus and are described in text, but they are not illustrated for clarity. B, Major spinocerebellar pathways carrying tactile and proprioceptive information to the cerebellum from the upper and lower parts of the body. Again, pathways from the head originate in the trigeminal nucleus but are not shown for clarity. A midsagittal view of the nervous system shows the levels of the spinal and brainstem cross sections in panels A and B. |
| page 106 |  | | page 107 |
| The sensory functions of various cutaneous sensory receptors have been studied in human subjects with a technique known as microneurography, in which a fine metal microelectrode is inserted into a nerve trunk in the arm or leg to record the action potentials from single sensory axons. When a recording can be made from a single sensory axon, the receptive field of the fiber is mapped. Most of the various types of sensory receptors that have been studied in experimental animals have also been found in humans with this technique. |
| After the receptive field of a sensory axon has been characterized, the electrode can be used to stimulate the same sensory axon. In these experiments the subject is asked to locate the perceived receptive field of the sensory axon, which turns out to be identical to the mapped receptive field. |
| The somatosensory pathways can also be classified by the type of information that they carry. Two broad functional categories are recognized, each of which subsumes several somatosensory submodalities. Fine discriminatory touch sensations include light touch, pressure, vibration, flutter (low-frequency vibration), and stretch or tension. The second major functional group of sensations is that of pain and temperature. Submodalities here include both noxious and innocuous
cold and warm sensations and mechanical and chemical pain. Itch is also closely related to pain and appears to be carried by particular fibers associated with the pain system.
|
| Of great importance experimentally, the afferent fibers that convey these somatosensory submodalities to the CNS are different sizes. Recall that the compound action potential recorded from a peripheral nerve (Chapter 5, Fig. 5-13 and Table 5-1) consists of a series of peaks, thus implying that the diameters of axons in a nerve are grouped rather than being uniformly distributed. Information about tactile sensations is carried primarily by large-diameter myelinated fibers in the Aα and Aβ classes, whereas pain and temperature information travels via small-diameter, lightly myelinated (Aδ) and unmyelinated (C) fibers. It is possible to block or selectively stimulate a class of axons of particular size, thereby allowing study of the different somatosensory submodalities in isolation.
|
| Low-Threshold Mechanosensory
|
| The skin is an important sensory organ and, not surprisingly, is richly innervated with a variety of afferents. We first consider the afferent types related to fine or discriminatory touch sensations. These afferents are related to what are called low-threshold mechanoreceptors. Nociceptor and thermoceptor innervation will be considered separately in a later section of this chapter.
|
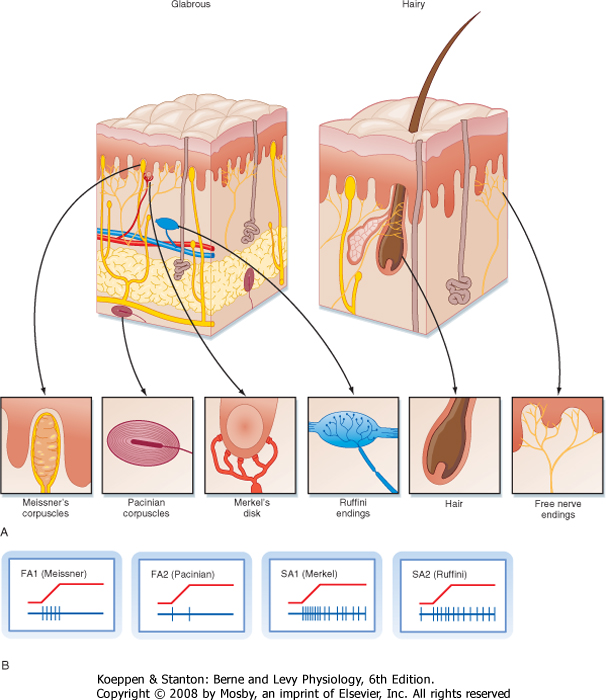
| To study the responsiveness of tactile receptors, a small-diameter rod or wire is used to press on a localized region of skin. With this technique, two basic types of responses may be seen when recording sensory afferent fibers: fast-adapting (FA) and slow-adapting (SA) responses (Fig. 7-2). They are present in similar quantities. FA fibers will show a short burst of action potentials when the rod first pushes down on the skin, but then they will cease firing despite continued application of the rod. They may also burst at the cessation of the stimulus (i.e., when the rod is lifted off). In contrast, SA units will start firing action potentials (or increase their firing rate) at the onset of the stimulus and continue to fire until the stimulus ends (Fig. 7-2).
|
| Both the FA and SA afferent classes can be subdivided on the basis of other aspects of their receptive fields, where receptive field is defined as the region of skin from which stimuli can evoke a response (i.e., change the firing of the afferent axon). Type 1 units have small receptive fields with well-defined borders. Particularly for glabrous skin (i.e., hairless skin, such as on the palms of the hands and soles of the feet), the receptive field has a circular or ovoid shape, within which there is relatively uniform and high sensitivity to stimuli that decreases sharply at the border (Fig. 7-3). Type 1 units, particularly SA1 units, respond best to edges. That is, a larger response is elicited from them when the edge of a stimulus cuts through their receptive field than when the entire receptive field is indented by the stimulus.
|
| Type 2 units have wider receptive fields with poorly defined borders and only a single point of maximal sensitivity, from which there is a gradual reduction in sensitivity with distance (Fig. 7-3). For comparison, a type 1 unit's receptive field typically will cover approximately four papillary ridges in the fingertip, whereas a type 2 unit will have a receptive field that covers most or all of a finger.
|
| Receptive Field Properties
|
| Thus, four main classes of low-threshold mechanosensitive afferents have been identified physiologically (FA1, FA2, SA1, and SA2). Peripherally, these axons may terminate as either free nerve endings or within a capsule made up of supporting cells.
|
| For glabrous skin, the four afferent classes have been associated with four specific types of histologically identified receptor capsules whose locations and physical structure help explain the firing properties of these sensory afferents. FA1 afferents terminate in Meissner's corpuscles, whereas SA1 afferents terminate in Merkel's disks. In both cases the capsule is located relatively superficially, either in the basal epidermis (Merkel) or just below the epidermis (Meissner) (Fig. 7-2). These capsules are small and oriented to detect stimuli pressing down on the skin surface just above them, thus allowing SA1 and FA1 afferents to have small receptive fields. For glabrous skin, SA2 afferents terminate in Ruffini's endings and FA2 afferents end in Pacinian corpuscles. Both these receptors lie deeper in the dermis and connective tissue and therefore are sensitive to stimuli applied over much larger territory. Both Pacinian and Meissner's capsules act to filter out slowly changing or steady stimuli, thus making these afferents selectively sensitive to changing stimuli.
|
| page 107 |  | | page 108 |
| Figure 7-2 Cutaneous mechanoreceptors and the response patterns of associated afferent fibers. A, Schematic views of glabrous (hairless) and hairy skin showing the arrangement of the various major mechanoreceptors. B, Firing patterns of the different cutaneous low-threshold mechanosensitive afferent fibers that innervate the various encapsulated receptors of the skin. (Traces in B are based on data from Johansson RS, Vallbo ÅB: Trends Neurosci 6:27, 1983.) |
| page 108 |  | | page 109 |
| Figure 7-3 Receptive field characteristics for type 1 and type 2 sensory afferents. Plots in the top row show the threshold level of force needed to evoke a response as a function of the distance across the receptive field. Receptive field size is shown on the hand below each plot. (Data from Johansson RS, Vallbo ÅB: Trends Neurosci 6:27, 1983.) |
| For hairy skin, the relationship between receptors and afferent classes is similar to that of glabrous skin. SA1 and SA2 fibers connect to Merkel's and Ruffini's endings, the same as for glabrous skin. Pacinian corpuscles
also underlie the properties of FA2 afferents; however, they are not found in hairy skin but, instead, are located in deep tissues surrounding muscles and blood vessels. There is not an exact analogue to the FA1 afferents. Rather, there are hair units, which are afferents whose free endings wrap around hair follicles (Fig. 7-2). Each such hair unit will connect with about 20 hairs to produce a large ovoid or irregularly shaped receptive field. These units are extremely sensitive to movement of even a single hair. There are also field units that respond to touch of the skin, but unlike FA1 units, they have large receptive fields.
|
| Several psychophysical and neural coding questions can be related to the receptive field properties and sensitivities of the various categories of afferents. For example, is the threshold of perception of tactile stimuli due to the sensitivity of the peripheral receptors or to central processes? In fact, by using microneurography, it is possible to show that a single spike in an FA1 afferent from the finger can be perceived, thus indicating that the receptors limit the sensitivity; however, for other skin regions, perception is more dependent on central factors such as attention.
|
| An important behavioral and clinical measure of somatosensory function is spatial acuity or two-point discrimination. Clinically, a doctor will apply two needle-like points simultaneously to the skin of a patient. The patient will generally perceive the points as two distinct stimuli as long as they are farther apart than some threshold distance, which varies across the body. The best discrimination (shortest threshold distance) is at the fingertips. Type 1 units underlie spatial acuity, which is not surprising given the smaller receptive fields of type 1 units than type 2 units; moreover, the threshold distance for a region of skin is most closely related to its density of type 1 units because these units have similarly sized receptive fields throughout the glabrous skin but their density falls off from fingertip to palm to forearm and this fall off correlates with the rise in threshold distance. Note that this variation in innervation density also matches the overall sensitivity of different skin regions to cutaneous stimuli.
|
| page 109 |  | | page 110 |
| The relationship of the firing rates in the various afferent classes to perceived stimulus quality is another important issue that has been addressed with microneurographic techniques. When a single SA fiber is stimulated with brief current pulses such that each pulse triggers a spike, a sensation of steady pressure is felt at the receptive field area of that fiber. As pulse frequency is intensified, an increase in pressure is perceived. Thus, the firing rate in SA fibers codes for the force of the tactile stimulus. As another example, when an FA fiber is repetitively stimulated, a sensation of tapping results first, and as the frequency of the stimulus is increased, the sensation
turns to one of vibration. Interestingly, in neither case does the stimulus change its qualitative character, for example, to a feeling of pain, as long as the stimulus activates only a particular fiber class. This is evidence that pain is a distinct submodality that uses a set of fibers distinct from those used by low-threshold mechanoreceptors.
|
| These findings illustrate an important principle of sensory systems called labeled line. The idea is that the quality (i.e., modality) of a particular sensation results from the fact that it is conveyed to the CNS by a specific set of afferents that have a distinct set of targets in the nervous system. Alterations in activity in these afferents will therefore change only quantitative aspects of the sensation. As will be seen in more detail later, the various somatosensory submodalities (i.e., information arising from FA and SA mechanoreceptors, proprioceptors, and nociceptors) appear to use relatively separate dedicated cell populations, even at relatively high levels of the CNS, such as the thalamus and primary somatosensory cortex.
|
| Axons of the peripheral nervous system (PNS) enter or leave the CNS through the spinal roots (or through cranial nerves). The dorsal root on one side of a given spinal segment is composed entirely of the central processes of dorsal root ganglion cells. The ventral root consists chiefly of motor axons, including α motor axons, γ motor axons (see Chapter 9), and at certain segmental levels, autonomic preganglionic axons (see Chapter 11).
|
| The pattern of innervation is determined during embryological development. In adults, a given dorsal root ganglion supplies a specific cutaneous region, which is called a dermatome. Many dermatomes become distorted during development, chiefly because of rotation of the upper and lower extremities as they are formed, but also because humans maintain an upright posture. However, the sequence of dermatomes can readily be understood if depicted on the body of a person in a quadrupedal position (Fig. 7-4).
|
| Although a dermatome receives its densest innervation from the corresponding spinal cord segment, collaterals of afferent fibers from the adjacent spinal segments also supply the dermatome. Thus, transection of a single dorsal root causes little sensory loss in the corresponding dermatome. Anesthesia of any given dermatome requires the interruption of several adjacent dorsal roots.
|
| A common disease that illustrates the dermatomal organization of the dorsal roots is shingles. Shingles is the result of reactivation of the herpes zoster virus, which typically causes chickenpox during the initial infection. During the initial infection the virus infects dorsal root ganglion cells, where it can remain latent for years to decades. When the virus reactivates, the cells of that particular dorsal root ganglion become infected, and the virus travels along the peripheral axon branches and gives rise to a painful or itchy rash that is confined to one side of the body (ends at the midline) in a dermatomal or belt-like distribution. |
| Figure 7-4 A, Dermatomes represented on a drawing of a person assuming a quadrupedal position. B, Sagittal view of the spinal cord showing the origin of nerves corresponding to each of the dermatomes shown in A. |
| page 110 |  | | page 111 |
| The trigeminal nuclear complex consists of four main divisions, three of which are sensory. The three sensory divisions (from rostral to caudal) are the mesencephalic, chief (or main) sensory, and spinal (or descending) trigeminal nuclei. The latter two are typical sensory nuclei in that the cell bodies contained in them are second-order neurons. The mesencephalic nucleus actually contains first-order neurons and thus is analogous to a dorsal root ganglion. The last division of the trigeminal complex is the motor nucleus of the trigeminal nerve, whose motor neurons project to skeletal muscles of the head via the trigeminal nerve (see Fig. 4-7, C-G). |
 |
| Within the dorsal roots, fibers are not randomly distributed. Rather, the large myelinated primary afferent fibers assume a medial position in the dorsal root, whereas the fine myelinated and unmyelinated fibers are more lateral. The large, medially placed afferent fibers enter the dorsal column, where they bifurcate to form rostrally and caudally directed branches.
These branches give off collaterals that terminate in the several neighboring segments. The rostral branch also ascends to the medulla as part of the dorsal column-medial lemniscus pathway. The axonal branches that terminate locally in the spinal cord gray matter transmit sensory information to neurons in the dorsal horn and also provide the afferent limb of reflex pathways (see Chapter 9).
|
| The arrangement of primary afferent fibers that supply the face is comparable to that of fibers that supply the body and is provided for primarily by fibers of the trigeminal nerve. Peripheral processes of neurons in the trigeminal ganglion pass through the ophthalmic, maxillary, and mandibular divisions of the trigeminal nerve to innervate dermatome-like regions of the face. These fibers carry both tactile information and pain and temperature information. The trigeminal nerve also innervates the teeth, the oral and nasal cavities, and the cranial dura mater.
|
| The central processes of trigeminal ganglion cells enter the brainstem at the midpontine level, which also corresponds to the level of the chief sensory trigeminal nucleus (nucleus of cranial nerve V). Some axons terminate in this nucleus (primarily large-caliber axons carrying the information needed for fine discriminative touch), whereas others (intermediate- and small-caliber axons that carry information about touch, as well as pain and temperature) form the spinal trigeminal tract, which descends through the medulla just lateral to the spinal trigeminal nucleus. As the tract descends, axons peel off and synapse in the nucleus.
|
| Proprioceptive information is also conveyed via the trigeminal nerve; however, in this unique case, the cell bodies of the first-order fibers are located within the CNS in the mesencephalic portion of the trigeminal nucleus. The central processes of these neurons terminate in the motor trigeminal nucleus (to subserve segmental reflexes equivalent to the segmental spinal cord reflexes-see Chapter 9), the reticular formation, and the chief sensory trigeminal nucleus.
|
| Central Somatosensory Pathways for Discriminatory Touch and Proprioception
|
| As may already be clear, information related to the different somatosensory submodalities travels, to a large extent, via separate pathways up the spinal cord and brainstem. For example, from the body, fine discriminatory touch information is conveyed by the dorsal column-medial lemniscus pathway, whereas pain, temperature, and crude touch information is conveyed by the anterolateral system.
|
| Proprioceptive information is transmitted by yet another route that partially overlaps with the dorsal column-medial lemniscal pathway. Note, however, that this functional segregation is not absolute, so, for example, there can be some recovery of discriminative touch ability after a lesion of the dorsal columns. The anterolateral system will be discussed in the section on pain because it is the critical pathway for that information. Here, the central pathways for discriminatory touch and proprioception are considered in detail.
|
| Dorsal Column-Medial Lemniscus Pathway
|
| This pathway is shown in its entirety in Figure 7-1, A. The dorsal columns are formed by ascending branches of the large myelinated axons of dorsal root ganglion cells (the first-order neurons). These axons enter at each spinal segmental level and travel rostrally up to the caudal medulla to synapse in one of the dorsal column nuclei: the nucleus gracilis, which receives information from the lower part of the body and leg, and the nucleus cuneatus, which receives information from the upper part of the body and arm. Note that in the dorsal columns and across the dorsal column nuclei there is a somatotopic representation of the body, with the legs represented most medially, followed by the trunk and then the upper limb. This somatotopy is a consequence of newly entering afferents being added to the lateral border of the dorsal funiculus as the spinal cord is ascended. Such somatotopic maps are present at all levels in the somatosensory system, at least through the primary sensory cortices.
|
| The dorsal column nuclei are located in the medulla and contain the second-order neurons of the pathway for discriminatory touch sensation. These cells respond similarly to the primary afferent fibers that synapse on them (see the earlier description of afferent types). The main differences between the responses of dorsal column neurons and primary afferent neurons are as follows: (1) dorsal column neurons have larger receptive fields because multiple primary afferent fibers synapse on a given dorsal column neuron, (2) dorsal column neurons sometimes respond to more than one class of sensory receptor because of the convergence of several different types of primary afferent fibers on the second-order neurons, and (3) dorsal column neurons often have inhibitory receptive fields that are mediated through local interneurons.
|
| page 111 |  | | page 112 |
| The axons of dorsal column nuclear projection neurons exit the nuclei and are referred to as the internal arcuate fibers as they sweep ventrally and then
medially to cross the midline at the same medullary level as the nuclei. Immediately after crossing the midline, these fibers form the medial lemniscus, which projects rostrally to the thalamus. Knowledge of this decussation level is clinically important because damage to the dorsal column-medial lemniscal pathway below this level, which includes all of the spinal cord, will produce loss of fine somatosensory discriminatory abilities on the same, or ipsilateral, side of the lesion, whereas lesions above this level will produce contralateral deficits. Moreover, because there is a clear somatotopic arrangement of fibers in the medial lemniscus, localized lesions cause selective loss of fine-touch sensations limited to specific body regions.
|
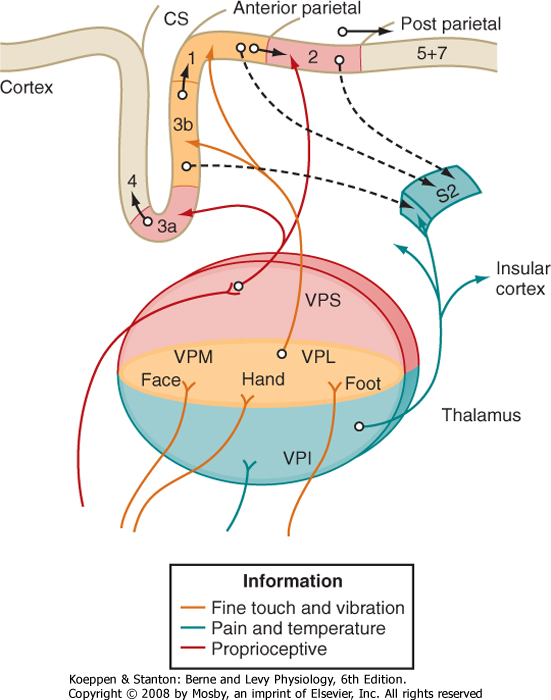
| The third-order neurons of the pathway are located in the ventral posterior lateral (VPL) nucleus of the thalamus and project to somatosensory areas of the cerebral cortex (Fig. 7-5).
|
| Figure 7-5 Diagram of connections from the somatosensory receiving nuclei of the thalamus to the somatosensory cortex of the parietal lobe. Note the parallel flow of different types of somatosensory information through the thalamus and onto the cortex. CS = central sulcus. Note: collectively areas 3a, 3b, 1, and 2 are referred to as S1. |
| The dorsal column-medial lemniscus pathway conveys information about fine-touch and vibratory sensations. This information is critical for many of the discriminatory tactile abilities that we have. For example, spatial acuity is lowered by damage to this pathway, and the ability to identify objects by their shape and texture can be lost by damage to this pathway. Clinically, one may test for impaired graphesthesia,
or the ability to recognize letters or numbers traced on the skin, or for loss of the ability to tell the direction of a line drawn across the skin. Importantly, some tactile function remains even after complete loss of the dorsal columns, and awareness and localization of nonnoxious tactile stimuli can still occur. Thus, at least some of the information carried by the dorsal column pathway is also conveyed by additional ascending pathways. In contrast to the severe deficits in discriminatory touch sensation, cutaneous pain and temperature sensations are unaffected by lesions of the dorsal columns. However, visceral pain is substantially diminished by damage to this pathway.
|
| Trigeminal Pathway for Fine-Touch Sensation from the Face
|
| Primary afferent fibers that supply the face, teeth, oral and nasal cavities, and cranial meninges synapse in several brainstem nuclei, including the main sensory nucleus and the spinal nucleus of the trigeminal nerve.
|
| The pathway through the main sensory nucleus resembles the dorsal column-medial lemniscus pathway. This sensory nucleus relays tactile information to the contralateral ventral posterior medial (VPM) thalamic nucleus by way of the trigeminothalamic tract. Third-order neurons in the VPM nucleus project to the facial area of the somatosensory cortex.
|
| Spinocerebellar and Proprioceptive Pathways
|
| Proprioceptors provide information about the positions and movement of parts of the body. In addition to being used for local reflexes (see Chapter 9), this information has two main targets, the cerebellum and the cerebral cortex. The cerebellum uses this information for its motor coordination functions. The information sent to the cortex is the basis for conscious awareness of our body parts (e.g., the position of our hand), which is referred to as kinesthesia.
|
| The major pathways by which somatosensory information is brought to the cerebellum are shown in Figure 7-1, B. These pathways carry both cutaneous and proprioceptive information to the cerebellum. For the trunk and lower part of the leg, the pathway starts with dorsal root ganglion cells whose axons synapse in (nucleus dorsalis) Clarke's column. The cells of Clarke's column send their axons into the ipsilateral lateral funiculus to form the dorsal spinocerebellar tract, which enters the cerebellum via the inferior cerebellar peduncle. The ventral spinocerebellar tract also provides somatosensory input from the lower limb to the cerebellum. Note the double decussation of the ventral spinocerebellar pathway (one decussation at the spinal cord levels and a second one in the cerebellar white matter). This double crossing highlights the general rule that each half of the cerebellum is functionally related to the ipsilateral side of the body.
|
| page 112 |  | | page 113 |
| To provide proprioceptive information from the lower limb to the cerebral cortex, the main axons of the dorsal spinocerebellar tract give off a branch in
the medulla that terminates in nucleus z, which is just rostral to the nucleus gracilis. The axons of cells from nucleus z then form part of the internal arcuate fibers and medial lemniscus and ascend to the VPL nucleus of the thalamus.
|
| The ascending somatosensory pathways to the cerebellum for the upper limb are simpler than those from the lower limb (Fig. 7-1, B). The route to the cerebellum starts with dorsal root ganglion fibers from the cervical spinal levels that ascend in the cuneate fasciculus to the external cuneate nucleus. The axons of the external cuneate then form the cuneocerebellar tract, which enters the cerebellum via its inferior peduncle.
|
| The route to the cerebral cortex for proprioceptive information from the upper limb is identical to that for discriminative touch: the dorsal column-medial lemniscal pathway, with a synapse in the cuneate nucleus and then in the VPL nucleus of the thalamus.
|
| For the head, proprioceptive input is carried by cells of the mesencephalic nucleus of the trigeminal nerve. Recall that the neurons in this nucleus are actually the cell bodies of the primary afferents that innervate stretch receptors in the muscles of mastication and in other muscles of the head. The central processes of these neurons project to the trigeminal motor nucleus for local reflexes or to the nearby reticular formation. Axons from these reticular formation neurons join the trigeminothalamic tract, which terminates in the VPM of the thalamus. There are also trigeminocerebellar pathways for conveying somatosensory (tactile and proprioceptive) information from the head to the cerebellum.
|
| THALAMIC AND CORTICAL SOMATOSENSORY AREAS
|
| The ventroposterior nuclear complex of the thalamus represents the main termination site for ascending somatosensory information in the diencephalon. It consists of two major nuclei, the VPL and VPM, and a smaller nucleus called ventral posterior inferior (VPI) (Fig. 7-5). The medial lemniscus forms the main input to the VPL nucleus, and the equivalent trigeminothalamic tract from the main sensory nucleus of the trigeminal nerve forms the main input to the VPM nucleus. These nuclei also receive weaker input from the spinothalamic or equivalent trigeminothalamic tracts, respectively. The VPI nucleus receives input from the spinothalamic tract.
|
| Single-unit recordings from the ventroposterior complex of nuclei have shown that the responses of many of the neurons in these nuclei to stimuli resemble those of first- and second-order neurons in the ascending tracts. The receptive fields of thalamic cells are small, but somewhat larger than those of primary afferent fibers. Moreover, the responses may be dominated by a particular type of sensory receptor. For example, VPL and VPM nuclei have cells whose receptive fields typically reflect input either from one type of cutaneous receptor (FA or SA) or from proprioceptive receptors, as expected from their dominant medial lemniscal input. In contrast, cells of the VPI and posterior nuclei show responses to activation of nociceptors, the main input to the spinothalamic pathway.
|
| Thalamic neurons often have inhibitory, as well as excitatory, receptive fields. The inhibition may actually take place in the dorsal column nuclei or in the dorsal horn of the spinal cord. However, inhibitory circuits are also situated within the thalamus. The VPL and VPM nuclei contain inhibitory interneurons (in primates, but not in rodents), and some of the inhibitory interneurons in the reticular nucleus of the thalamus project into the VPL and VPM nuclei. The inhibitory neurons intrinsic to the VPL and VPM nuclei and in the reticular nucleus use γ-aminobutyric acid (GABA) as their inhibitory neurotransmitter.
|
| One difference between neurons in the VPL and VPM nuclei and sensory neurons at lower levels of the somatosensory system is that thalamic neuron excitability depends on the stage of the sleep-wake cycle and on the presence or absence of anesthesia. During a state of drowsiness or during barbiturate anesthesia, thalamic neurons tend to undergo an alternating sequence of excitatory and inhibitory postsynaptic potentials. The alternating bursts of discharges in turn intermittently excite neurons in the cerebral cortex. Such patterns of excitation and inhibition result in an α rhythm or in spindling on the electroencephalogram. This alternation of excitatory and inhibitory postsynaptic potentials during these two states may reflect the level of excitation of thalamic neurons by excitatory amino acids that act at non-N-methyl-d-aspartate (NMDA) and NMDA receptors. It may also reflect inhibition of the thalamic neurons by recurrent pathways through the reticular nucleus.
|
| Thalamic neuron receptive fields are on the side of the body contralateral to the neuron, and the receptive field locations vary systematically across the ventroposterior nuclear complex. That is, the VPL and VPM nuclei are somatotopically organized such that the lower limb is represented most laterally and the upper limb most medially in the VPL nucleus and the head is represented even more medially in the VPM nucleus. Moreover, the fact that thalamic neurons often receive input from only one class of receptor suggests that there are multiple somatotopic maps laid out across the ventroposterior nuclear complex. That is, there appear to be separate somatotopic maps for SA, FA, and proprioceptive and pain sensations laid out across the ventroposterior nuclear complex.
|
| These maps are not randomly interspersed. As already mentioned, pain sensation is largely mapped across the VPI nucleus. In addition, the cutaneous receptors appear to drive cells located in a central "core" region of the VPL-VPM complex, whereas proprioceptive information is directed to cells that form a "shell" (VPS) around this core. This parallel flow of information into thalamus and then onto the cortex is diagramed in Figure 7-5.
|
| page 113 |  | | page 114 |
| The spinothalamic tract also projects to other thalamic regions, including the posterior nucleus and the
central lateral nucleus of the intralaminar complex of the thalamus. The intralaminar nuclei of the thalamus are not somatotopically organized, and they project diffusely to the cerebral cortex, as well as to the basal ganglia (see Chapter 9). The projection of the central lateral nucleus to the S-I cortex may be involved in arousal of this part of the cortex and in selective attention.
|
| Third-order sensory neurons in the thalamus project to the somatosensory cortex. The details of this projection pattern are shown in Figure 7-5. The main somatosensory receiving areas of the cortex are called the S-I and S-II areas. The S-I cortex (or primary somatosensory cortex) is located on the postcentral gyrus, and the S-II cortex (secondary somatosensory cortex) is in the superior bank of the lateral fissure (Fig. 7-5).
|
| As previously discussed, the S-I cortex, like the somatosensory thalamus, has a somatotopic organization. The S-II cortex also contains a somatotopic map, as do several other less understood areas of the cortex. In the S-I cortex, the face is represented in the lateral part of the postcentral gyrus, above the lateral fissure. The hand and the rest of the upper extremity are represented in the dorsolateral part of the postcentral gyrus and the lower extremity on the medial surface of the hemisphere. A map of the surface of the body and face of a human on the postcentral gyrus is called a sensory homunculus. The map is distorted because the volume of neural tissue devoted to a body region is proportional to the density of its innervation. Thus, in humans, the perioral area, the thumb, and other digits take up a disproportionately large expanse of cortex relative to their size.
|
| The sensory homunculus is an expression of place coding of somatosensory information. A locus in the S-I cortex encodes the location of a somatosensory stimulus on the surface of the body or face. For example, the brain knows that a certain part of the body has been stimulated because certain neurons in the postcentral gyrus are activated.
|
| The S-I cortex has several morphological and functional subdivisions, and each subdivision has a somatotopic map. These subdivisions were originally described by Brodmann, and they were based on the arrangements of neurons in the various layers of the cortex, as seen in Nissl-stained preparations. The subdivisions are therefore known as Brodmann areas 3a, 3b, 1, and 2 (see Chapter 10). Cutaneous input dominates in areas 3b and 1, whereas muscle and joint input (proprioceptive) dominates in areas 3a and 2. Thus, separate cortical zones are specialized for the processing of tactile and proprioceptive information.
|
| Within any particular area of S-I cortex, all the neurons along a line perpendicular to the cortical surface have similar response properties and receptive fields. The S-I cortex is thus said to have a columnar organization. A comparable columnar organization has also been demonstrated for other primary sensory receiving areas, including the primary visual and auditory cortices (see Chapter 8). Nearby cortical columns in the S-I cortex may process information for different sensory modalities. For example, the cutaneous information that reaches one cortical column in area 3b may come from FA mechanoreceptors, whereas the information that reaches a neighboring column might originate from SA mechanoreceptors.
|
| Besides being responsible for the initial processing of somatosensory information, the S-I cortex also begins higher-order processing, such as feature extraction. For example, certain neurons in area 1 respond preferentially to a stimulus that moves in one direction across the receptive field, but not in the opposite direction (Fig. 7-6). Such neurons presumably contribute to the perceptual ability to recognize the direction of an applied stimulus and could help detect slippage of an object being grasped by the hand.
|
| Effects of Lesions of the Somatosensory Cortex
|
| A lesion of the S-I cortex in humans produces sensory changes similar to those produced by a lesion of the somatosensory thalamus. However, usually only a part of the cortex is involved, and thus the sensory loss may be confined, for example, to the face or to the leg, depending on the location of the lesion with respect to the sensory homunculus. The sensory modalities most affected are discriminative touch and position sense. Graphesthesia and stereognosis (i.e., the ability to recognize objects, such as coins and keys, as they are handled) are particularly disturbed. Pain and thermal sensation may be relatively unaffected, although loss of pain sensation may follow cortical lesions. Conversely, cortical lesions can result in a central pain state that resembles thalamic pain (see below).
|
| PAIN AND TEMPERATURE SENSATION
|
| The sensations of pain and temperature are related and often grouped together because they are mediated by overlapping sets of receptors and are conveyed by the same types of fibers in the PNS and the same pathways in the CNS. One consequence of these labeled lines is that pain sensations, in particular, are not just due to stronger activation of touch pathways, as might naively be thought. This difference is borne out experimentally because if SA afferents, for example, are stimulated more and more frequently, the sensation of tactile pressure becomes stronger, but not painful.
|
| Nociceptors and Primary Afferents
|
| The axons that carry painful and thermal sensations are members of the relatively slowly conducting Aδ and C classes. However, not all Aδ and C axons carry pain and temperature information; some respond to light touch in a manner similar to what was described for low-threshold mechanoreceptors.
|
| page 114 |  | | page 115 |
| Figure 7-6 Feature extraction by cortical neurons. The responses were recorded from a neuron in the somatosensory cortex of a monkey. The direction of a stimulus was varied, as shown by the arrows in the drawing. Note that the responses were greatest when the stimulus moved in the direction from UW to RF and least from RW to UF. F, fingers; R, radial side; U, ulnar side; W, wrist. (From Costanzo RM, Gardner EP: J Neurophysiol 43:1319, 1980.) |
| Unlike the case for low-threshold mechanoreceptors in which morphologically distinct receptors correspond to response properties, the Aδ and C axons conveying pain and temperature information appear to end mostly as "free nerve endings." (This description
is not entirely accurate because the endings are mostly, but not entirely, covered by Schwann cells.) Despite the lack of distinct morphological specialization associated with their endings, Aδ and C axons constitute a heterogeneous population that is differentially sensitive to a variety of tissue-damaging or thermal stimuli (or both). This ability to sense tissue-damaging stimuli (mechanical, thermal, or chemical) is mediated by what are called nociceptors. These receptors share some features with low-threshold mechanoreceptors but are distinct in many ways, such as the ability to become sensitized (see later). Indeed, there appear to be a significant number of C fibers that are silent or unresponsive to any stimuli until first sensitized.
|
| The first functional distinction that may be made in the pain system is between Aδ and C axons. Aδ axons conduct signals faster than C fibers do and are thought to underlie what is called first pain, whereas C fibers are responsible for second pain. Thus, after a damaging stimulus, one first feels an initial sharp, pricking, highly localized sensation (first pain), followed by a duller, more diffuse, burning sensation (second pain). Experiments in which Aδ or C fibers were selectively activated demonstrated that activity in Aδ fibers produces sensations similar to first pain and that activity in C fibers produces second pain-like sensations.
|
| Each fiber class, in turn, forms a heterogeneous group with regard to sensitivity to stimuli. Thus, afferents are classified according to both size and their sensitivity to mechanical, thermal, and chemical stimuli. Fibers may have a low or high threshold to mechanical stimulation or be completely insensitive to it. Thermal sensitivity has been classified as responsiveness to warmth, noxious heat, cool, and noxious cold. Note that 43°C and 15°C are the approximate limits above and below which, respectively, thermal stimuli are sensed as painful. Chemical sensitivity to a variety of irritating compounds has been tested, including capsaicin (found in chili peppers), mustard oil, and acids.
|
| Afferent fibers may be sensitive to one or more types of stimuli and have been named accordingly. For example, C fibers sensitive only to high-intensity (damaging) mechanical stimuli are called C mechanosensitive fibers, whereas those sensitive to heat and mechanical stimuli are labeled C mechanoheat-sensitive fibers (also called polymodal fibers). Other identified fiber types include Aδ and C cold-sensitive, Aδ mechanosensitive, and mechanoheat-sensitive fibers. Thus, there is quite a variety of afferent types; however, the most common afferent type is the C polymodal fiber, which accounts for nearly half of the cutaneous C fibers. Surprisingly, the second most common type is the mechanoheat-insensitive afferent (i.e., an afferent that is not sensitive to noxious stimuli until sensitized-see later).
|
| page 115 |  | | page 116 |
| Because all these fibers begin as free nerve endings, their distinct sensitivities must be the result of distinct membrane receptors. These proteins, however, have been difficult to identify, in large part because the low density of receptors makes purification of these proteins difficult (contrast single nerve endings scattered within a patch of skin to the numbers of rod cell outer segments in the retina, each of which is packed with discs filled with rhodopsin molecules). Nevertheless, over the past decade or so potential candidates have been identified via a variety of approaches. The receptor that binds capsaicin (the molecule in chili peppers responsible for their spiciness) has been identified,
and either it or one of a family of related proteins has been found to be expressed in populations of dorsal root ganglion cells. These proteins belong to the TRP (transient receptor potential) protein family and are currently the most likely candidates for being the transducers of thermal sensations.
|
| Members of the TRP protein family were first identified in Drosophila and were found to be part of the phototransduction process in Drosophila photoreceptors. Thus, the name (TRP) refers to the fact that a mutation in the gene leads to a transient depolarizing response to a light stimulus instead of the normal sustained response. On the basis of sequence homology, a number of genes encoding TRP proteins have been found in mammals (27 in humans alone), which are currently divided into seven subfamilies. TRP channels are cation permeable and have a structure similar to voltage-gated K+ channels. They are homotetramers or heterotetramers. Each subunit has six transmembrane domains. TRP proteins appear to have a variety of functions (e.g., phototransduction, chemotransduction, and mechanotransduction) and are expressed in a number of cell types. Those listed in Table 7-1 appear to act as temperature sensors with distinct thermal sensitivities that span the range of physiologically relevant temperatures. |
 |
| It is important to note that many ion channels (and other proteins, e.g., enzymes) are sensitive to temperature; however, in the case of TRP channels, temperature is acting directly as the gating mechanism. The temperatures at which specific TRP channels are active are indicated by arrows in Figure 7-7, where the direction of each arrow indicates which temperatures cause greater activation. For comparison, Figure 7-7 also plots the firing rates of several thermosensitive fibers as a function of temperature. Note how the response ranges of the afferents can overlap with those of the heat-sensitive channels. The cold fibers, however, show firing over a wider range than any one TRP channel does. One possible explanation for this discrepancy is that dorsal root ganglion cells may express multiple classes of TRP, which would enable
them to respond over a wider range of physiological temperatures.
|
| As with thermal transducers, the transducer proteins for noxious mechanical stimulation have not been definitively identified in humans; however, at least some are likely to be homologues of proteins identified in Caenorhabditis elegans that belong to the DEG/ENaC (degenerin/epithelial Na+ channel) family. These are Na+ channels that are not voltage gated but are blocked by amiloride. The exact transduction mechanism is not known; however, two hypotheses are that the channel senses and is gated by tension in the cell membrane and that the channel is attached to the cytoskeleton intracellularly and fibers of the extracellular matrix and senses tension via these connections.
|

|
| Figure 7-7 Temperature dependence of firing rates in different thermosensitive afferents. Below the firing curves are shown the ranges over which the different TRP channels are activated. The direction of increasing activation is indicated by arrow in each case. Note how in some cases the range over which an afferent is active corresponds well to the activation range of a single TRP channel, thus suggesting that the afferent would need to express only a single type of channel. In other cases, the afferent firing range suggests that multiple TRP channels would be needed to underlie the complete responsiveness of the afferent. |
|
Table 7-1.
TRP Family Proteins Involved in Thermal Transduction |
| Receptor Protein | Threshold or Temperature Range for Activation (°C) | Other Characteristics |
| TRPV1 | >42 | Activated by capsaicin |
| TRPV2 | >52 | |
| TRPV3 | 34-38 | Activated by camphor |
| TRPV4 | 27-34 | |
| TRPM8 | <25 | Activated by menthol |
| TRPA1 | <18 | Activated by mustard oil |
|
The fourth letter in the name identifies the subfamily and was chosen because of the first member of the subfamily identified: V, vanilloid; M, melastatin; A, ankyrin-like. Each of the proteins listed is expressed in at least some dorsal root ganglion cells, but they are also expressed in other cell types.
|
| page 116 |  | | page 117 |
| As with the low-threshold mechanoreceptors for innocuous touch sensations, activation of the various
nociceptor transduction proteins leads to a generator potential that causes spiking of the afferent, which transmits information to the CNS. In addition, activation of nociceptors also leads to the local release of various chemical compounds, including tachykinins (substance P [SP]) and calcitonin gene-related protein (CGRP). These substances and others released from the damaged cells cause neurogenic inflammation (edema and redness of the surrounding skin).
|
| In addition to causing a local reaction, these substances may serve to activate the insensitive or silent nociceptors mentioned earlier such that they can henceforth respond to any subsequent damaging stimuli. Sensitization of silent nociceptors has been suggested to underlie allodynia (elicitation of painful sensations by stimuli that were innocuous before an injury) and hyperalgesia (increase in the level of pain felt to already painful stimuli).
|
| Spinal Cord Gray Matter and Trigeminal Nucleus
|
| The central portion of the Aδ and C axons carrying pain and temperature information from the body terminates in the dorsal horn of the spinal cord. The Aδ fibers target lamina I, V, and X of the gray matter, whereas the C fibers terminate in lamina I and II. The distinct termination patterns of the Aδ and C fibers in the spinal cord suggests that the messages they are carrying to the CNS are kept separated, and this is consistent with our ability to feel two distinct types of pain.
|
| The primary afferent termination patterns in the spinal cord are also important because they may help determine the possible interactions that pain fibers can have with other afferents and with descending control systems (see later). Indeed, the gate control theory of pain refers to the phenomenon that innocuous stimuli, such as rubbing a hurt area, can block or reduce painful sensations. Such stimulation activates the large-diameter (Aα and Aβ) fibers, and their activity leads to release of GABA and other neurotransmitters by interneurons within the dorsal horn. GABA then acts by both presynaptic and postsynaptic mechanisms to shut down the activity of spinothalamic tract cells. Presynaptically, GABA activates both GABAA and GABAB receptors, which leads to partial depolarization of the presynaptic terminal and blocking of Ca++ channels, respectively. Both actions will decrease release of transmitter by the afferent terminal and thereby lessen excitation of the tract cell (see Chapter 6 section on presynaptic inhibition).
|
| Elderly people are sometimes susceptible to a condition of chronic pain known as trigeminal neuralgia. People with this condition experience spontaneous episodes of severe, often lancinating pain in the distribution of one or more branches of the trigeminal nerve. Frequently, the pain is triggered by weak mechanical stimulation in the same region. A major contributing factor to this painful state appears to be mechanical damage to the trigeminal ganglion by an artery that impinges on the ganglion. Surgical displacement of the artery can often resolve the condition. |
 |
| Nociceptive and thermoreceptive information that originates from regions of the head is processed in a fashion similar to that for the trunk and limbs. The primary afferent fibers of nociceptors and thermoreceptors in the head enter the brainstem through the trigeminal nerve (some also enter through the facial, glossopharyngeal, and vagus nerves). Of note, the trigeminal distribution includes both tooth and headache pain. These fibers then descend through the brainstem to the upper cervical spinal cord via the spinal tract of the trigeminal nerve. Some mechano-receptive
afferent fibers also join the spinal tract of the trigeminal nerve. Axons in the spinal tract synapse on second-order neurons in the spinal nucleus of the trigeminal nerve.
|
| The central pain pathways include the spinothalamic, spinoreticular, and spinomesencephalic tracts. The spinothalamic tract is the most important sensory pathway for somatic pain and thermal sensations from the body (Fig. 7-1, A). It also contributes to tactile sensation. The spinothalamic tract originates from second-order neurons located in the spinal cord (primarily laminae I and IV to VI). The axons of these cells cross to the opposite side of the cord at or near to their level of origin. They then ascend to the brain in the ventral part of the lateral funiculus and subsequently through the brainstem to the thalamus, where they terminate on third-order neurons, as described earlier. Spinothalamic cells conveying pain and temperature target the VPI portion of the ventroposterior complex (although some also end in the VPL), the posterior nucleus, and the intralaminar nuclei of the thalamus. Nociceptive signals are then forwarded to several cortical areas, including not only the somatosensory cortex but also cortical areas that are involved in affective responses, such as the cingulate gyrus and the insula, which have limbic system functions (Fig. 7-5).
|
| Most spinothalamic tract cells receive excitatory input from nociceptors in the skin, but many can also be excited by noxious stimulation of muscle, joints, or viscera. Few receive input only from viscera. Effective cutaneous stimuli include noxious mechanical, thermal (hot or cold), and chemical stimuli. Thus, different spinothalamic tract cells respond in a manner appropriate for signaling noxious, thermal, or mechanical events.
|
| page 117 |  | | page 118 |
| Figure 7-8 A, Responses of a wide-dynamic range or multireceptive spinothalamic tract cell. B, Responses of a high-threshold spinothalamic tract cell. The figures indicate the excitatory (plus signs) and inhibitory (minus signs) receptive fields. The graphs show the responses to graded intensities of mechanical stimulation. Brush stimulus is a camel's hair brush repeatedly stroked across the receptive field. Pressure is applied by attachment of an arterial clip to the skin. This is a marginally painful stimulus to a human. Pinch is achieved by attachment of a stiff arterial clip to the skin and is distinctly painful. Squeeze is applied by compressing a fold of skin with forceps and is damaging to the skin. |
| Some nociceptive spinothalamic tract cells receive convergent excitatory input from several different classes of cutaneous sensory receptors. For example, a given spinothalamic neuron may be activated weakly by tactile stimuli but more powerfully by noxious stimuli (Fig. 7-8). Such neurons are called wide-dynamic range cells because they are activated by
stimuli with a wide range of intensities. Wide-dynamic range neurons signal mainly noxious events; weak responses to tactile stimuli appear to be ignored by the higher centers. However, in certain pathological conditions, these neurons may be sufficiently activated by tactile stimuli to evoke a sensation of pain, possibly as a result of activity in sensitized afferents that were previously silent. This would explain some pain states in which the activation of mechanoreceptors causes pain (mechanical allodynia). Other spinothalamic tract cells are activated only by noxious stimuli. Such neurons are often called high-threshold or nociceptive-specific cells (Fig. 7-8, B).
|
| Because cells signaling visceral input also typically convey information from cutaneous receptors, the brain may misidentify the source of the pain. This phenomenon is called referred pain. A typical example is when the heart muscle becomes ischemic and pain is felt in the chest wall and left arm.
|
| page 118 |  | | page 119 |
| Neurotransmitters released by nociceptive afferents that activate spinothalamic tract cells include the excitatory amino acid glutamate and any of several peptides, such as SP, CGRP, and vasoactive intestinal polypeptide (VIP). Glutamate appears to act as a fast transmitter by its action on non-NMDA excitatory amino acid receptors. However, with repetitive stimulation,
glutamate can also act through NMDA receptors. Peptides appear to act as neuromodulators. For example, through a combined action with an excitatory amino acid such as glutamate, SP can produce a long-lasting increase in the responses of spinothalamic tract cells; this enhanced responsiveness is called central sensitization. CGRP seems to increase release of SP and prolong the action of SP by inhibiting its enzymatic degradation.
|
| Spinothalamic tract cells often have inhibitory receptive fields. Inhibition may result from weak mechanical stimuli, but usually the most effective inhibitory stimuli are noxious ones. The nociceptive inhibitory receptive fields may be very large and may include most of the body and face (Fig. 7-8, A). Such receptive fields may account for the ability of various physical manipulations, including transcutaneous electrical nerve stimulation and acupuncture, to suppress pain. Neurotransmitters that can inhibit spinothalamic tract cells include the inhibitory amino acids GABA and glycine, as well as monoamines and the endogenous opioid peptides.
|
| Spinoreticular tract neurons frequently have large, sometimes bilateral receptive fields, and effective stimuli include noxious ones. These dorsal horn neurons target multiple regions in the medullary and pontine reticular formation. The reticular formation, which projects to the intralaminar complex of the thalamus and then to wide areas of the cerebral cortex, is involved in attentional mechanisms and arousal (see Chapter 10). The reticular formation also gives rise to descending reticulospinal projections, which contribute to the descending systems that control transmission of pain.
|
| Many cells of the spinomesencephalic tract respond to noxious stimuli, and the receptive fields may be small or large. The terminations of this tract are in several midbrain nuclei, including the periaqueductal gray, which is an important component of the endogenous analgesia system. Motivational responses may also result from activation of the periaqueductal gray matter. For example, stimulation in the periaqueductal gray matter can cause vocalization and aversive behavior. Information from the midbrain is relayed not only to the thalamus but also to the amygdala, a part of the limbic system. This provides one of several pathways by which noxious stimuli can trigger emotional responses.
|
| Pain and temperature information originating from the face and head is conveyed along analogous ascending central pathways, as is such information from the body. Neurons in the spinal trigeminal nucleus transmit pain and temperature information to specific nuclei (VPM, VPI) of the contralateral thalamus via the ventral trigeminothalamic tract, which runs in close association with the medial lemniscus. The spinal nucleus also projects to the intralaminar complex and other thalamic nuclei in a fashion similar to that of the spinothalamic tract. The thalamic nuclei in turn project to the somatosensory cerebral cortex for sensory discrimination of pain and temperature and to other cortical regions responsible for motivational-affective responses.
|
| Effects of Interruption of the Spinothalamic Tract and Lesions of the Thalamus on Somatosensory Sensation
|
| When the spinothalamic tract and accompanying ventral spinal cord pathways are interrupted, both the sensory-discriminative and the motivational-affective components of pain are lost on the contralateral side of the body. This result motivated development of the surgical procedure known as anterolateral cordotomy, which was used to treat pain in many individuals, especially those suffering from cancer. This operation is now used infrequently because of improvements in drug therapy and because pain often returns months to years after an initially successful cordotomy. Return of pain may reflect either an extension of the disease or the development of a central pain state. In addition to the loss of pain sensation, anterolateral cordotomy produces loss of cold and warmth sensation on the contralateral side of the body. Careful testing may reveal a minimal tactile deficit as well, but the intact sensory pathways of the dorsal part of the spinal cord provide sufficient tactile information that any loss caused by interruption of the spinothalamic tract is insignificant.
|
| Destruction of the VPL or VPM nuclei diminishes sensation on the contralateral side of the body or face. The sensory qualities that are lost reflect those that are transmitted mainly by the dorsal column-medial lemniscus pathway and its trigeminal equivalent. The sensory-discriminative component of pain sensation is also lost. However, the motivational-affective component of pain is still present if the medial thalamus is intact. Presumably, pain persists because of the spinothalamic and spinoreticulothalamic projections to this part of the thalamus. In some individuals, a lesion of the somatosensory thalamus results in a central pain state known as thalamic pain. However, pain that is indistinguishable from thalamic pain can also be produced by lesions in the brainstem or cortex.
|
| Pain sometimes occurs in the absence of nociceptor stimulation. This type of pain is most likely to occur after damage to peripheral nerves or to parts of the CNS that are involved in transmitting nociceptive information. Pain caused by damage to neural structures is called neuropathic pain. Neuropathic pain states include peripheral neuropathic pain, which may follow damage to a peripheral nerve, and central neuropathic pain, which sometimes occurs after damage to CNS structures.
|
| page 119 |  | | page 120 |
| Examples of pain secondary to damage to a peripheral nerve are causalgia and phantom limb pain. Causalgia may develop after traumatic damage to a peripheral nerve. Even though evoked pain is reduced, severe pain may develop in the area innervated by the damaged nerve. This pain may be very difficult to treat, even with strong analgesic drugs. The pain is
caused in part by spontaneous activity that develops in dorsal root ganglion cells; such activity may be attributed to up-regulation of sodium channels. In some cases the pain seems to be maintained by sympathetic neural activity because a sympathetic nerve block may alleviate the pain. Sympathetic involvement may relate to the sprouting of damaged sympathic postganglionic axons into the dorsal root ganglia, and it may be accompanied by up-regulation of adrenoreceptors in primary afferent neurons. Phantom limb pain follows traumatic amputation in some individuals. Such phantom pain is clearly not caused by the activation of nociceptors in the area in which pain is felt because these receptors are no longer present.
|
| Lesions of the thalamus or at other levels of the spinothalamocortical pathway may cause central pain, which is a severe, spontaneous pain. However, interruption of the nociceptive pathway by the same lesion may simultaneously prevent or reduce the pain evoked by peripheral stimulation. The mechanism of such trauma-induced pain caused by neural damage is poorly understood. The pain appears to depend on changes in the activity and response properties of more distant neurons in the nociceptive system.
|
| CENTRIFUGAL CONTROL OF SOMATOSENSATION
|
| Sensory experience is not just the passive detection of environmental events. Instead, it more often depends on exploration of the environment. Tactile cues are sought by moving the hand over a surface. Visual cues result from scanning targets with the eyes. Thus, sensory information is often received as a result of activity in the motor system. Furthermore, transmission in pathways to the sensory centers of the brain is regulated by descending control systems. These systems allow the brain to control its input by filtering the incoming sensory messages. Important information can be attended to and unimportant information can be ignored.
|
| The tactile and proprioceptive somatosensory pathways are regulated by descending pathways that originate in the S-I and motor regions of the cerebral cortex. For example, corticobulbar projections to the dorsal column nuclei help control the sensory input that is transmitted by the dorsal column-medial lemniscus pathway.
|
| Of particular interest is the descending control system that regulates the transmission of nociceptive information. This system presumably suppresses excessive pain under certain circumstances. For example, it is well known that soldiers on the battlefield, accident victims, and athletes in competition often feel little or no pain at the time that a wound occurs or a bone is broken. At a later time, pain may develop and become severe. Although the descending regulatory system that controls pain is part of a more general centrifugal control system that modulates all forms of sensation, the pain control system is so important medically that it is distinguished as a special system called the endogenous analgesia system.
|
| Several centers in the brainstem and pathways descending from these centers contribute to the endogenous analgesia system. For example, stimulation in the midbrain periaqueductal gray, the locus caeruleus, or the medullary raphe nuclei inhibits nociceptive neurons at the spinal cord and brainstem level, including spinothalamic and trigeminothalamic tract cells (Fig. 7-9, A). Other inhibitory pathways originate in the sensorimotor cortex, the hypothalamus, and the reticular formation.
|
| The endogenous analgesia system can be subdivided into two components: one component uses endogenous opioid peptides as neurotransmitters and the other does not. Endogenous opioids are neuropeptides that activate one of several types of opiate receptors. Some of the endogenous opioids include enkephalin, dynorphin, and β-endorphin. Opiate analgesia can generally be prevented or reversed by the narcotic antagonist naloxone. Therefore, naloxone is frequently used to determine whether analgesia is mediated by an opioid mechanism.
|
| The opioid-mediated endogenous analgesia system can be activated by the exogenous administration of morphine or other opiate drugs. Thus, one of the oldest medical treatments of pain depends on the triggering of a sensory control system. Opiates typically inhibit neural activity in nociceptive pathways. Two sites of action have been proposed for opiate inhibition, presynaptic and postsynaptic (Fig. 7-9, B). The presynaptic action of opiates on nociceptive afferent terminals is thought to prevent the release of excitatory transmitters such as SP. The postsynaptic action of opiates produces an inhibitory postsynaptic potential. How can an inhibitory neurotransmitter activate descending pathways? One hypothesis is that the descending analgesia system is under tonic inhibitory control by inhibitory interneurons in both the midbrain and the medulla. The action of opiates would inhibit the inhibitory interneurons and thereby disinhibit the descending analgesia pathways.
|
| Some endogenous analgesia pathways operate by neurotransmitters other than opioids and thus are unaffected by naloxone. One way of engaging a nonopioid analgesia pathway is through certain forms of stress. The analgesia thus produced is a form of stress-induced analgesia.
|
| page 120 |  | | page 121 |
| Figure 7-9 A, Some of the neurons thought to play a role in the endogenous analgesia system. Neurons in the midbrain periaqueductal gray activate the raphe-spinal tract, which in turn inhibits nociceptive spinal neurons, such as those of the spinothalamic tract (STT). Interneurons containing opioid substances are involved in the system at each level. B, Possible presynaptic and postsynaptic sites of action of enkephalin (Enk). The presynaptic action might prevent the release of substance P (Sub P.) from nociceptors. (Redrawn from Henry JL. In Porter R, O'Connor M [eds]: Ciba Foundation Symposium 91. London, Pitman, 1982.) |
| Many neurons in the raphe nuclei use serotonin as a neurotransmitter. Serotonin can inhibit nociceptive neurons and presumably plays an important role in the endogenous analgesia system. Other brainstem neurons release catecholamines, such as norepinephrine and epinephrine, in the spinal cord. These catecholamines also inhibit nociceptive neurons; therefore, catecholaminergic neurons may contribute to the endogenous analgesia system. Furthermore, these monoamine neurotransmitters interact with endogenous opioids. Undoubtedly, many other substances are involved in the analgesia system. In addition, there
is evidence for the existence of endogenous opiate antagonists that can prevent opiate analgesia.
|
| page 121 |  | | page 122 |
- Sensory neurons have cell bodies in sensory nerve ganglia: dorsal root ganglia for neurons innervating the body and cranial nerve ganglia for neurons innervating the face, oral and nasal cavities, and dura, except for proprioceptive neurons, which are in the trigeminal mesencephalic nucleus. They connect peripherally to a sensory receptor and centrally to second-order neurons in the spinal cord or brainstem.
- Skin contains low-threshold mechanoreceptors, thermoreceptors, and nociceptors. Muscle, joints, and viscera have mechanoreceptors and nociceptors. Low-threshold mechanoreceptors may be rapidly or slowly adapting. Thermoreceptors include cold and warm receptors. Aδ and C nociceptors detect noxious mechanical, thermal, and chemical stimuli and may be sensitized by release of chemical substances from damaged cells. Peripheral release of substances, such as peptides, from nociceptors themselves may contribute to inflammation.
- Large primary afferent fibers enter the dorsal funiculus through the medial part of the dorsal root; collaterals synapse in the deep dorsal horn, intermediate zone, and ventral horn. Small primary afferent fibers enter the dorsolateral fasciculus through the lateral part of the dorsal root; collaterals synapse in the dorsal horn.
- Ascending branches of large primary afferent fibers synapse on second-order neurons in the dorsal column nuclei. These second-order neurons project in the medial lemniscus to the contralateral thalamus and synapse on third-order neurons of the VPL nucleus. The equivalent trigeminal pathway is relayed by the main sensory nucleus and contralateral VPM nucleus.
- The dorsal spinal cord pathways signal the sensations of flutter-vibration, touch-pressure, and proprioception. They also contribute to visceral sensation, including visceral pain.
- The spinothalamic tract includes nociceptive, thermoreceptive, and tactile neurons; its cells of origin are mostly in the dorsal horn, and the axons cross, ascend in the ventrolateral funiculus, and synapse in the VPL, VPI, and posterior and intralaminar nuclei of the thalamus. The equivalent trigeminal pathway is relayed by the spinal trigeminal nucleus and projects to the contralateral VPM and intralaminar nuclei.
- The spinothalamic relay in the VPL and VPI nuclei helps account for the sensory-discriminative aspects of pain. Parallel nociceptive pathways in the ventrolateral funiculus are the spinoreticular and spinomesencephalic tracts; these tracts and the spinothalamic projection to the medial thalamus contribute to the motivational-affective aspects of pain.
- Referred pain is explained by convergent input to spinothalamic tract cells from the body wall and from viscera.
- The VPL and VPM nuclei are somatotopically organized and contain inhibitory circuits. These nuclei contain multiple somatotopic maps, one for each somatosensory submodality. The somatosensory cortex includes the S-I and S-II regions; these regions are also somatotopically organized.
- The S-I cortex contains columns of neurons with similar receptive fields and response properties. Some S-I neurons are involved in feature extraction.
- Transmission in somatosensory pathways is regulated by descending control systems. The endogenous analgesia system regulates nociceptive transmission, and it uses transmitters such as endogenous opioid peptides, norepinephrine, and serotonin.
|
 |
|