| 10 Higher Functions of the Nervous System
|
| Interactions between different parts of the cerebral cortex and between the cerebral cortex and other parts of the brain are responsible for many of the higher functions that characterize humans. The neural basis for some of these higher functions is discussed in this chapter.
|
| The cerebral cortex in humans occupies a volume of about 600 cm3 and has a surface area of 2500 cm2. The surface of the cortex is highly convoluted and folded into ridges known as gyri. Gyri are separated by grooves called sulci (if shallow) or fissures (if deep). This folding greatly increases the surface area of cortex that can be fit into the limited and fixed volume that exists within the skull. Indeed, most of the cortex cannot be seen from the brain surface because of this folding (Fig. 10-1).
|
| The cerebral cortex can be divided into the left and right hemispheres and subdivided into a number of lobes (Fig. 10-1), including the frontal, parietal, temporal, and occipital lobes. These lobes are named for the overlying bones of the skull. The frontal and parietal lobes are separated by the central sulcus; they are separated from the temporal lobe by the lateral fissure. The occipital and parietal lobes are separated (on the medial surface of the hemisphere) by the parietooccipital fissure (Fig. 10-1). Buried within the lateral fissure is another lobe, the insula (Fig. 4-7A). The limbic lobe is formed by the cortex on the medial aspect of the hemisphere that borders on the brainstem. Part of the limbic lobe, the hippocampal formation, is folded into the parahippocampal gyrus of the temporal lobe and cannot be seen from the surface of the brain.
|
| Activity in the two hemispheres of the cerebral cortex is coordinated by interconnections through the cerebral commissures. The bulk of the neocortex on the two sides is connected through the massive corpus callosum (Fig. 10-1). Parts of the temporal lobes connect through the anterior commissure, and the hippocampal formations on the two sides communicate through the hippocampal commissure (which is formed between the fornices on the two sides as they approximate each other at the back of the septum pellucidum and pass under the corpus callosum).
|
| Functions of the Lobes of the Cerebral Cortex
|
| Specific functions of the cerebral cortex can be associated with different lobes of the cerebral hemispheres.
|
| One of the main functions of the frontal lobe is motor behavior. As discussed in Chapter 9, the motor, premotor, cingulate motor, and supplementary motor areas are located in the frontal lobe, as is the frontal eye field. These areas are crucial for planning and executing motor behavior. Broca's area, essential for the generation of speech, is located in the inferior frontal gyrus of the dominant hemisphere for human language (almost always the left hemisphere, as explained later). In addition, the prefrontal cortex in the rostral part of the frontal lobe plays a major role in personality and emotional behavior.
|
| Bilateral lesions of the prefrontal cortex may be produced either by disease or by surgically induced frontal lobotomy. Such lesions produce deficits in attention, difficulty in planning and problem solving, and inappropriate social behavior. Aggressive behavior is also reduced, and the motivational-affective component of pain is lost, although pain sensation remains. Frontal lobotomies are rarely performed today because improved drug therapies have become available for mental illness and chronic pain.
|
| The parietal lobe contains the somatosensory cortex and the adjacent parietal association cortex (see Chapter 7). This lobe is involved in the processing and perception of sensory information. Connections with the frontal lobe allow somatosensory information to affect voluntary motor activity. Visual information from the occipital lobe reaches the parietal association cortex and the frontal lobe, where it also helps guide voluntary movements. Somatosensory information can also be transferred to language centers, such as Wernicke's area, in the dominant hemisphere, as described later. The parietal lobe in the nondominant hemisphere is involved in determining spatial context, as shown by the effects of specific lesions (see Chapters 7 and 9).
|
| page 201 |  | | page 202 |
| Figure 10-1 Lateral and medial views of the left hemisphere of the human cerebrum with the major features labeled and the lobes indicated by color. R, G, B, and S indicate, respectively, the rostrum, genu, body, and splenium of the corpus callosum. (From Haines DE [ed]: Fundamental Neuroscience for Basic and Clinical Applications, 3rd ed. Philadelphia, Churchill Livingstone, 2006.) |
| page 202 |  | | page 203 |
| The primary function of the occipital lobe is visual processing and perception (see Chapter 8). Connections
to the frontal eye fields affect eye movements, and a projection to the midbrain assists in the control of convergent eye movements, pupillary constriction, and accommodation, all of which occur when the eyes adjust for near vision.
|
| The temporal lobe has many different functions. One of these functions is hearing, which depends on processing and perception of information related to sounds (see Chapter 8). Another function is processing of vestibular information. Several visual areas have been discovered in the temporal lobe; hence, this lobe is also involved in higher-order visual processing (see Chapter 8). For example, the infratemporal cortex, on its inferior surface, is involved in the recognition of faces. In addition, Meyer's loop, which forms part of the optic pathway, passes through the temporal lobe. Therefore, temporal lobe lesions can damage vision in part of the visual fields. Similarly, some of Wernicke's area, essential for the understanding of language, lies in the posterior region of the temporal lobe.
|
| The medial temporal lobe belongs to the limbic system, which participates in emotional behavior and regulates the autonomic nervous system (see Chapter 11). The hippocampal formation is involved in learning and memory (see later).
|
| Neocortical Layering and Subdivisions
|
| The cerebral cortex can be subdivided phylogenetically into the archicortex, paleocortex, and neocortex. In humans, 90% of the cortex is neocortex.
|
| The functions of the different lobes of the cerebral cortex have been defined both from the effects of lesions produced by disease or by surgical interventions to treat disease in humans and from experiments on animals. In another approach, the physical manifestations of epileptic seizures have been correlated with the brain locations that give rise to seizures (epileptic seizure foci). For example, epileptic foci in the motor cortex cause movements on the contralateral side; the exact movements relate to the somatotopic location of the seizure focus. Seizures that originate in the somatosensory cortex cause an epileptic aura in which a sensation is experienced. Similarly, seizures that start in the visual cortex cause a visual aura (scintillations, colors), those in the auditory cortex cause an auditory aura (humming, buzzing, ringing), and those in the vestibular cortex cause a feeling of spinning. Complex behavior results from seizures that originate in the temporal lobe association areas; in addition, a malodorous aura may be perceived if the olfactory cortex is involved (uncinate fit). |
 |
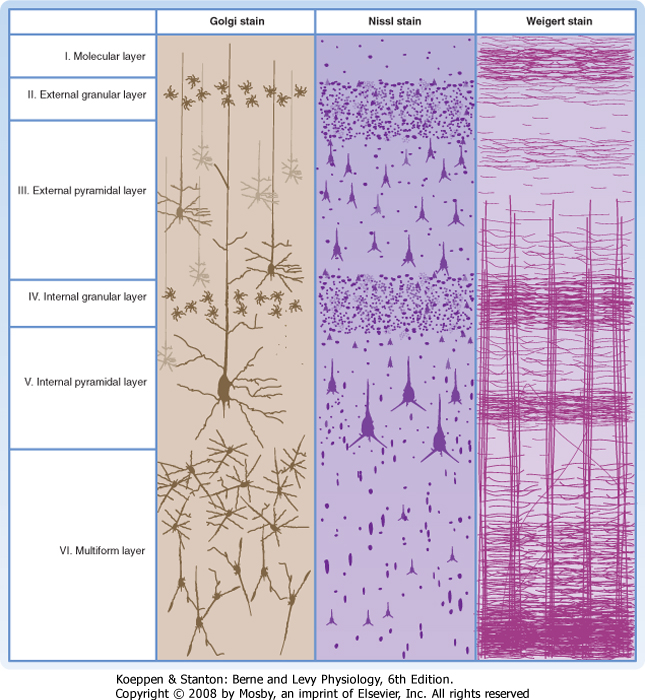
| The different phylogenetic subdivisions of the cerebral cortex can be recognized on the basis of their layering pattern. The neocortex is generally characterized
by the presence of six cortical layers (Fig. 10-2). In contrast, the archicortex has only three layers and the paleocortex has four to five layers.
|
| Cell Types in the Neocortex
|
| A number of different cell types have been described in the neocortex (Fig. 10-2). Pyramidal cells are the most abundant cell type and account for approximately 75% of neocortical neurons. Stellate cells and various other types of nonpyramidal cells make up the balance. Pyramidal cells have a large triangular cell body, a long apical dendrite directed toward the cortical surface, and several basal dendrites. The axon emerges from the cell body opposite the apical dendrite, and those from the larger pyramidal cells project into the subcortical white matter. The axon may give off collaterals as it descends through the cortex. Pyramidal cells use an excitatory amino acid (glutamate or aspartate) as their neurotransmitter. Stellate cells, often called granule cells, are interneurons. They have a small soma and numerous branched dendrites, although many will have an apical dendrite and thus look like small pyramidal cells. Some are excitatory interneurons; these cells are abundant in layer IV of the cortex (see below). Their axons remain in the same cortical region and frequently ascend toward the supragranular layers. Other stellate cells are inhibitory interneurons that use γ-aminobutyric acid (GABA) as their neurotransmitter.
|
| Cytoarchitecture of Cortical Layers
|
| Each of the six layers of the neocortex has a characteristic cellular content (Fig. 10-2). Layer I (molecular layer) has few neuronal cell bodies; instead, it contains mostly axon terminals and synapses on dendrites. Layer II (external granular layer) contains mostly stellate cells. Layer III (external pyramidal layer) consists mostly of small pyramidal cells. Layer IV (internal granular layer) contains mostly stellate cells, including the excitatory type. Layer V (internal pyramidal layer) is dominated by large pyramidal cells. These cells are the main source of cortical efferents to most subcortical regions. Layer VI (multiform layer) contains pyramidal, fusiform, and other types of cells. This layer is also an important origin of cortical efferents, those that target thalamic nuclei.
|
| Cortical Afferent and Efferent Fibers
|
| Thalamocortical afferent fibers from thalamic nuclei that have specific (topographically mapped) cortical projections end chiefly in layers III, IV, and VI. Neurons in other thalamic nuclei (particularly those relaying input from the reticular formation) project diffusely and terminate in layers I and VI.
|
| Several nonthalamic, diffusely projecting nuclei (including the basal nucleus of Meynert, the locus coeruleus, and the dorsal raphe nucleus) project to all cortical layers. These projections, along with those projecting from the thalamus to layers I and VI, modulate cortical activity globally, perhaps in conjunction with changes in state (e.g., sleep or waking).
|
| page 203 |  | | page 204 |
| Figure 10-2 A small area of neocortex stained by three different methods. The Nissl stain (center) shows the cell bodies of all neurons and reveals how different types are distributed among the six layers. The Golgi stain (left) shows only a sample of the neuronal population but reveals details of their dendrites. The Weigert stain for myelin (right) demonstrates vertically oriented bundles of axons entering and leaving the cortex and horizontally coursing fibers that interconnect neurons within a layer. (From Brodmann K: Vergleichende Lokalisation lehre der Grosshirnrinde in ihren prinzipien Dargestellt auf Grund des Zellenbaues. Leipzig, Germany, JA Barth, 1909.) |
| The cortical efferent axons originate from pyramidal cells. The pyramidal cells of layers II and III project to other cortical areas, either ipsilaterally or contralaterally, via the corpus callosum. The pyramidal cells of layer V project in many descending pathways and have synaptic targets in the spinal cord, brainstem, striatum, and thalamus. The pyramidal cells of layer VI form corticothalamic projections to the thalamic nuclei with specific cortical projections. Reciprocal thalamocortical and corticothalamic interconnections are likely to make important contributions to the electroencephalogram (EEG) (see later).
|
| page 204 |  | | page 205 |
| Regional Variations in Neocortical Structure
|
| On the basis of differences in cytoarchitecture, a number of subdivisions of the neocortex can be recognized. Most of the cortex is composed of six readily distinguishable layers. The primary and premotor areas are sometimes said to be agranular cortex. This is a misnomer because all cortical areas, including these motor areas, have similar percentages of pyramidal and nonpyramidal cells (≈75% versus 25%). However, in the frontal motor areas, the nonpyramidal cell bodies do not group in a manner that leads to the formation of distinct "granular" layers. Moreover, local inhibitory interneurons play an important role in somatotopic organization of the primary motor cortex (see Chapter 9).
|
| The primary motor cortex, in fact, is characterized by the presence of the largest pyramidal neurons in the cortex, called Betz cells. These enormous cells have axons that contribute to the corticospinal tracts and whose soma size (diameter >150 μm) is necessary for the metabolic maintenance of so much axoplasm. (Note that most corticospinal axons are from pyramidal cells because Betz cell axons account for less than 5% of all corticospinal fibers.)
|
| Another type of cortex has a very prominent layer IV and thus is called granular cortex. Dominated as it is by the stellate cells seen in layer IV of Figure 10-2, it is specialized for processing afferent input. Therefore, this kind of cortex is found in the primary sensory receiving areas: the somatosensory cortex (SI), the primary auditory cortex, and the primary visual (striate) cortex. The striate cortex is given its name because of a particularly prominent horizontal sheet of axons in layer IV known as the stripe of Gennari.
|
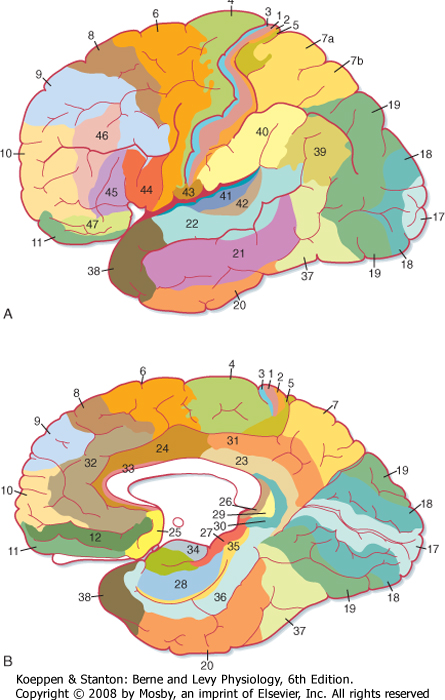
| Most of the other regions of cortex show less dramatic variations and often seem to grade from one type of layer morphology to the next as one looks at adjacent areas of cortex. On the basis of such an extensive cytoarchitectural analysis, Brodmann divided the cortex into 52 discrete areas (Fig. 10-3). Commonly referred to areas include Brodmann's areas 3, 1, and 2 (the SI cortex of the postcentral gyrus); area 4 (the primary motor cortex of the precentral gyrus); area 6 (the premotor and supplementary motor cortex); areas 41 and 42 (the primary auditory cortex on the superior temporal gyrus); and area 17 (the primary visual cortex mostly on the medial surface of the occipital lobe). Detailed studies have confirmed that the Brodmann areas are, in fact, distinctly different, both with respect to their interconnections and with respect to their functions, but more recent work has shown that there is some plasticity, both in the size of the areas and in their internal organization (see later).
|
| Archicortex and Paleocortex
|
| About 10% of the human cerebral cortex is archicortex and paleocortex. The archicortex has a three-layered structure; the paleocortex has four to five layers. The paleocortex is located at the border between the archicortex and neocortex.
|
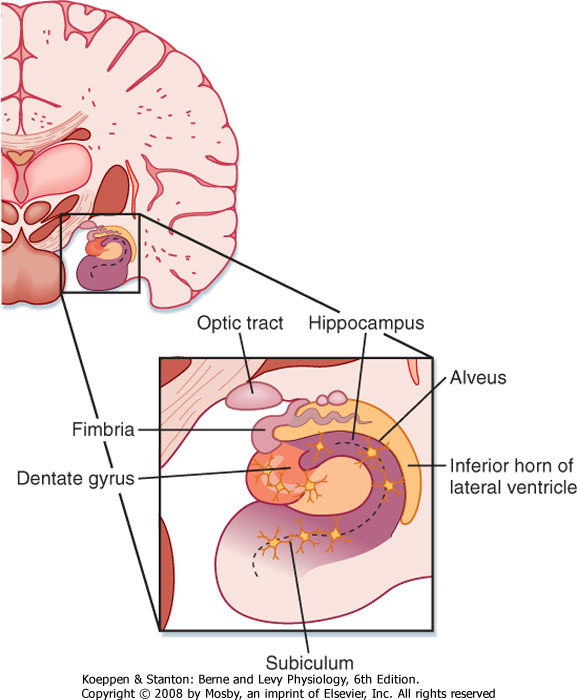
| In humans the hippocampal formation is part of the archicortex. It is folded into the temporal lobe and can be viewed only when the brain is dissected. The hippocampal formation consists of several parts, including the hippocampus (Ammon's horn or cornu ammonis), the dentate gyrus, and the subiculum. These divisions are well demarcated in a cross section through the hippocampal formation (Fig. 10-4).
|
| The hippocampus has three layers: the molecular, pyramidal cell, and polymorphic layers. They resemble layers I, V, and VI in the neocortex. The folding of the hippocampus imparts an inverted appearance because the white matter is at the surface of the lateral ventricle (Fig. 10-4). The white matter covering the hippocampus is called the alveus, which contains hippocampal afferent and efferent fibers. The axons in the alveus continue into a nerve fiber bundle called the fimbria; the fimbria is continuous with the fornix.
|
| The hippocampal formation receives its main neural input from the entorhinal cortex of the parahippocampal gyrus. Important, generally reciprocal connections are formed between the pyramidal cells of the hippocampus and (1) the septal nuclei and mammillary body by way of the fornix and (2) the contralateral hippocampal formation by way of the fornix and the hippocampal commissure. The hippocampus is a major component of Papez's circuit (see Chapter 11).
|
| HIGHER FUNCTIONS OF THE NERVOUS SYSTEM
|
| An EEG is a recording of neuronal electrical activity that can be made from the cerebral cortex via electrodes placed on the skull. In an electrocorticogram, electrical activity of the cortex is recorded via electrodes placed on the surface of the brain. These are both called field potentials because they detect the electrical field generated by large groups of relatively distant neurons. The EEG waves are derived from the excitatory and inhibitory synaptic potentials that occur in cortical neurons as a result of thalamocortical and other input, and they are produced chiefly by extracellular currents that flow vertically across the cortex during the generation of synaptic potentials in the pyramidal cells. The potentials recorded as the EEG are relatively large (around 100 μV) and reflect the activity of many pyramidal cells, which are arranged with their apical dendrites aligned in parallel to form a dipole sheet. One pole of this sheet is oriented toward the cortical surface and the other toward the subcortical white matter. Note that the sign of an EEG wave does not in itself indicate whether pyramidal cells are being excited or inhibited. For instance, a negative EEG potential may be generated at the surface of the skull (or cortex) by excitation of apical dendrites or by inhibition near the somas. Conversely, a positive EEG wave can be produced by inhibition of apical dendrites or by excitation near the somas.
|
| page 205 |  | | page 206 |
| Figure 10-3 Brodmann's areas in the human cerebral cortex. (Redrawn from Crosby EC et al: Correlative Anatomy of the Nervous System. New York, Macmillan, 1962.) |
| Although a brief EEG wave is sometimes referred to as a spike, this term does not refer to action potentials because the extracellular currents associated with action potentials are too small, fast, and asynchronous to be recorded with EEG electrodes.
|
| In human studies, the EEG is recorded from a grid of standard recording sites. Thus, EEGs can be recorded from approximately the same sites at different times from one individual or from analogous sites in different subjects. The EEG is an important diagnostic tool in clinical neurology and is particularly useful in patients with epilepsy.
|
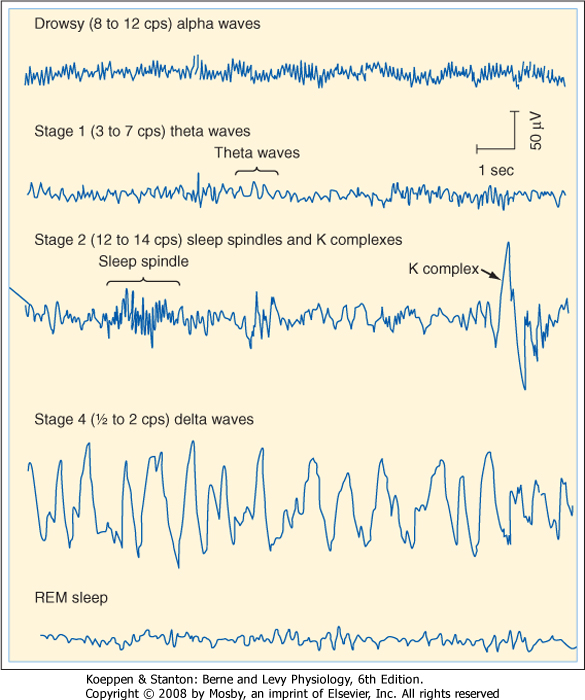
| A normal EEG consists of waves of various frequencies. The dominant frequencies depend on several factors, including the state of wakefulness, the age of the subject, the location of the recording electrodes, and the absence or presence of drugs or disease. When a normal awake adult is relaxed with the eyes closed, the dominant frequencies of the EEG recorded over the parietal and occipital lobes are about 8 to 13 Hz, the alpha rhythm. If the subject is asked to open his eyes, the EEG becomes less synchronized and the dominant frequency increases to 13 to 30 Hz, which is called the beta rhythm. The delta (0.5 to 4 Hz) and theta (4 to 7 Hz) rhythms are observed during sleep (see the following discussion) (Fig. 10-5).
|
| page 206 |  | | page 207 |
| Figure 10-4 The hippocampal formation is found on the medial aspect of the temporal lobe, and it protrudes into the inferior horn of the lateral ventricle. Its major components are the hippocampus, the dentate gyrus, and the subiculum, all of which are three-layered archicortex. The fimbria is the major output pathway from the hippocampal region to the mammillary body and the septal nuclei. |
| An EEG change, called a cortical evoked potential, can be elicited by a stimulus. A cortical evoked potential
is best recorded from the part of the skull located over the cortical area being activated. For example, a visual stimulus results in an evoked potential that can be recorded best over the occipital bone, whereas a somatosensory evoked potential is recorded most effectively near the junction of the frontal and parietal bones. Evoked potentials reflect the activity in large numbers of cortical neurons. They may also reflect activity in subcortical structures.
|
| Evoked potentials are small in comparison to the size of the EEG waves. However, their apparent size can be enhanced by a process called signal averaging. In this process, the stimulation is repeated and EEGs are recorded during each trial. With each repetition of the stimulus, the evoked potential occurs at a fixed interval after the stimulus. However, the underlying EEG may show a positive or a negative deflection on different trials during the time of occurrence of the evoked potential. In signal averaging, evoked potentials are electronically averaged. The random temporal association of the EEG waves with the stimulus results in their cancellation, whereas the evoked potentials sum.
|
| Evoked potentials are used clinically to assess the integrity of a sensory pathway, at least to the level of the primary sensory receiving area. These potentials can be recorded in comatose individuals, as well as in infants too young to permit a sensory examination. The initial parts of the auditory evoked potential actually reflect activity in the brainstem; therefore, this evoked potential can be used to assess the function of brainstem structures. |
| Sleep and wakefulness are among the many functions of the body that show circadian (about 1 day) periodicity. The sleep-wake cycle has an endogenous periodicity
of about 25 hours, but it normally becomes entrained to the day-night cycle. However, the entrainment can be disrupted when the subject is isolated from the environment or shifts time zones (jet lag).
|
| Characteristic changes in the EEG can be correlated with changes in the behavioral state during the sleep-wake cycle. Beta wave activity dominates in an awake, aroused individual. The EEG is said to be desynchronized; it displays low-voltage, high-frequency activity. In relaxed individuals with their eyes closed, the EEG is dominated by alpha waves (Fig. 10-5). A person falling asleep passes sequentially through four stages of slow-wave sleep (called stages 1 through 4) over a period of 30 to 45 minutes (Fig. 10-5). In stage 1, alpha waves are interspersed with lower-frequency waves (3 to 7 Hz) called theta waves. In stage 2, the EEG slows further, but the slow-wave activity is interrupted by sleep spindles, which are bursts of activity at 12 to 14 Hz, and by large K complexes (large, slow potentials). Stage 3 sleep is associated with delta waves, which occur at frequencies of 0.5 to 2 Hz, and with occasional sleep spindles. Stage 4 is characterized by delta waves.
|
| During slow-wave sleep the muscles of the body relax, but the posture is adjusted intermittently. The heart rate and blood pressure decrease and gastrointestinal motility increases. The ease with which individuals can be awakened decreases progressively as they pass through these sleep stages. As individuals awaken, they pass through the sleep stages in reverse order.
|
| page 207 |  | | page 208 |
| Figure 10-5 EEG during drowsiness and stages 1, 2, and 4 of slow-wave (non-rapid eye movement [non-REM]) sleep and REM sleep. (Modified from Shepherd GM: Neurobiology. London, Oxford University Press, 1983.) |
| The purpose of sleep is still unclear. However, it must have a high value because so much of life is spent in sleep and lack of sleep can be debilitating. Medically important disorders of the sleep-wake cycle include insomnia, bed-wetting, sleepwalking, sleep apnea, and narcolepsy. |
 |
| About every 90 minutes slow-wave sleep changes to a different form of sleep, called rapid eye movement (REM) sleep. In REM sleep, the EEG again becomes desynchronized. The low-voltage, fast activity of REM sleep resembles that seen in the EEG from an aroused subject (Fig. 10-5, bottom trace). The similarity of the EEG to that of an awake individual and the difficulty awaking the person have suggested the term paradoxical sleep for this type of sleep. Muscle tone is completely lost, but phasic contractions occur in a number of muscles, most notably the eye muscles. The resulting rapid eye movements are basis of the name for this type of sleep. Many autonomic changes also take place. Temperature regulation is lost, and meiosis occurs. Penile erection may occur during this type of sleep. Heart rate, blood pressure, and respiration
change intermittently. Several episodes of REM sleep occur each night. Although it is difficult to arouse a person from REM sleep, internal arousal is common. Most dreams occur during REM sleep.
|
| The proportion of slow-wave (non-REM) to REM sleep varies with age. Newborn children spend about half of their sleep time in REM sleep, whereas the elderly have little REM sleep. About 20% to 25% of the sleep of young adults is REM sleep.
|
| The mechanism of sleep is incompletely understood. Stimulation in the brainstem reticular formation in a large region known as the reticular activating system causes arousal and low-voltage, fast EEG activity. Sleep was once thought to be caused by a reduced level of activity in the reticular activating system. However, substantial data, including the observations that anesthesia of the lower brainstem results in arousal and that stimulation in the medulla near the nucleus of the solitary tract can induce sleep, suggest that sleep is an active process. Investigators have tried to relate sleep mechanisms to brainstem networks that use particular neurotransmitters, including serotonin, norepinephrine, and acetylcholine, because manipulations of the levels of these transmitters in the brain can affect the sleep-wake cycle. However, a detailed neurochemical explanation of the neural mechanisms of sleep is not yet available.
|
| The source of circadian periodicity in the brain appears to be the suprachiasmatic nucleus of the hypothalamus. This nucleus receives projections from the retina, and its neurons seem to form a biological clock that adapts to the light-dark cycle. Destruction of the suprachiasmatic nucleus disrupts a number of biological rhythms, including the sleep-wake cycle.
|
| Cerebral Dominance and Language
|
| page 208 |  | | page 209 |
| The EEG becomes abnormal in a variety of pathological circumstances. For example, during coma the EEG is dominated by delta activity. Brain death is defined by a maintained flat EEG. |
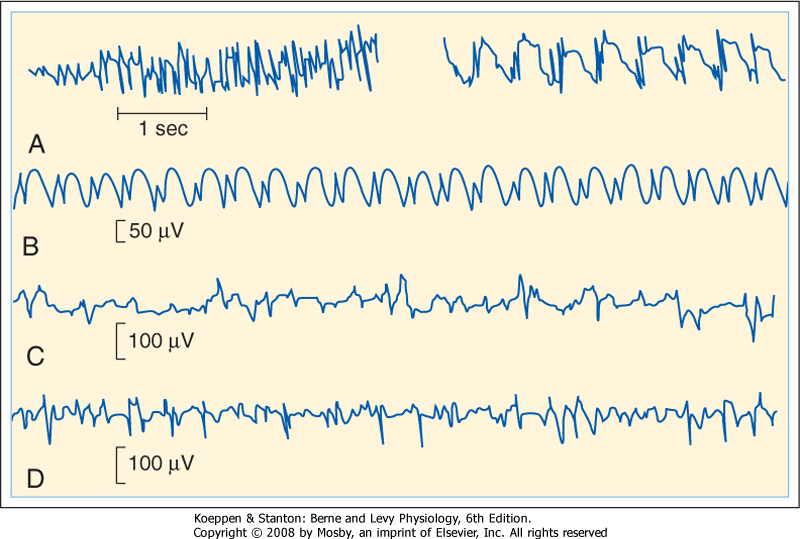
| Epilepsy commonly causes EEG abnormalities. There are several forms of epilepsy, and examples of EEG patterns from some of these types of epilepsy are shown in Figure 10-6. Epileptic seizures can be either partial or generalized. |
| One form of partial seizures originates in the motor cortex and results in localized contractions of contralateral muscles. The contractions may then spread to other muscles; such spread follows the somatotopic sequence of the motor cortex (see Chapter 9). This stereotypical progression is called a Jacksonian march. Complex partial seizures (which may occur in psychomotor epilepsy) originate in the limbic structures of the temporal lobe and result in illusions and semipurposeful motor activity. During and between focal seizures, scalp recordings may reveal EEG spikes (Fig. 10-6, C and D). |
| Generalized seizures involve wide areas of the brain and loss of consciousness. Two major types of seizures are petit mal and grand mal seizures. In petit mal epilepsy, consciousness is lost transiently, and the EEG displays spike and wave activity (Fig. 10-6, B). In grand mal seizures, consciousness is lost for a longer period, and the individual may fall to the ground if standing when the seizure starts. The seizure begins with a generalized increase in muscle tone (tonic phase), followed by a series of jerky movements (clonic phase). The bowel and bladder may be evacuated. The EEG shows widely distributed seizure activity (Fig. 10-6, A). |
| EEG spikes that occur between full-blown seizures are called interictal spikes. Similar events can be studied experimentally. These spikes arise from abrupt, long-lasting depolarizations, called depolarization shifts, that trigger repetitive action potentials in cortical neurons. These depolarization shifts may reflect several changes in epileptic foci. Such changes include regenerative Ca++-mediated dendritic action potentials in cortical neurons and a reduction in inhibitory interactions in cortical circuits. Electrical field potentials and the release of K+ and excitatory amino acids from hyperactive neurons may also contribute to the increased cortical excitability. |
 |
|
| Figure 10-6 EEG abnormalities in several forms of epilepsy. A, EEG during the tonic (left) and clonic (right) phases of a grand mal seizure. B, Spike and wave components of a petit mal seizure. C, EEG in temporal lobe epilepsy. D, A focal seizure. (Redrawn from Eyzaguirre C, Fidone SJ: Physiology of the Nervous System, 2nd ed. St Louis, Mosby, 1975.) |
| In most people, the left cerebral hemisphere is the dominant hemisphere with respect to language. This dominance has been demonstrated (1) by the effects of lesions of the left hemisphere, which may produce deficits in language function (aphasia), and (2) by the transient aphasia (inability to speak or write) that
results when a short-acting anesthetic is introduced into the left carotid artery. Lesions of the right hemisphere and injection of anesthetic into the right carotid artery do not usually affect language substantially. For example, left-handedness reflects a sensorimotor dominance of the right hemisphere, but for the majority of left-handed individuals, the left hemisphere is still dominant for language. Differences in the size of an area called the planum temporale, which is located in the floor of the lateral fissure, correlate with language dominance. The left planum temporale is usually larger than that of the right hemisphere.
|
| Several areas in the left hemisphere are involved in language. Wernicke's area is a large area centered in the posterior part of the superior temporal gyrus behind the auditory cortex. Another important language area, Broca's area, is in the posterior part of the inferior frontal gyrus, close to the face representation of the motor cortex. Damage to Wernicke's area results in a receptive aphasia in which the person has difficulty understanding spoken and written language; however, speech production remains fluent, if meaningless. Conversely, a lesion in Broca's area causes expressive aphasia. Individuals with expressive aphasia have difficulty in speech and in writing, although they can understand language relatively well.
|
| page 209 |  | | page 210 |
| A person with receptive aphasia may not have auditory or visual impairment and one with expressive aphasia may have normal motor control of the muscles
responsible for speech or writing. Thus, aphasia does not depend on a deficit of sensation or motor skill; rather, it is an inability to translate language-encoded sensory information into concepts and/or vice versa. Thus, the terms "motor aphasia" and "sensory aphasia" are misleading. However, lesions in the dominant hemisphere may be large enough to result in mixed forms of aphasia, as well as sensory changes or paralysis of some of the muscles used to express language. For example, a lesion of the face representation portion of the motor cortex would result in an inability to manipulate the motor apparatus needed for speaking (vocal cords, tongue, lips) and, consequently, unclear speech because of dysarthria, a mechanical deficit. Such an individual would, however, be able to write if the motor cortex for the upper limb were unaffected.
|
| Interhemispheric Transfer
|
| The two cerebral hemispheres can function somewhat independently, as in the case of language function. However, information must be transferred between the hemispheres to coordinate activity on the two sides of the body. In other words, each hemisphere must know what the other is doing. Much of the information transferred between the two hemispheres is transmitted through the corpus callosum, although some is transmitted through other commissures (e.g., the anterior commissure or the hippocampal commissure).
|
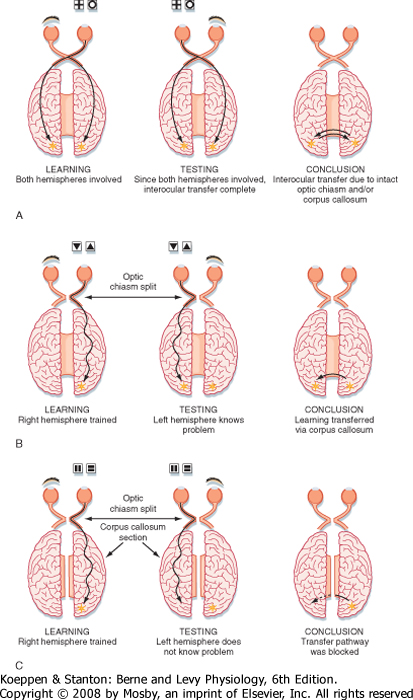
| An experiment that shows the importance of the corpus callosum for interhemispheric transfer of information is illustrated in Figure 10-7, A. An animal with an intact optic chiasm and corpus callosum and with the left eye closed learns a visual discrimination task (Fig. 10-7, A). The information is transmitted to both hemispheres through bilateral connections made by the optic chiasm or through the corpus callosum, or both. When the animal is tested with the left eye open and the right eye closed (Fig. 10-7, A, center), the task can still be performed because both hemispheres have learned the task. If the optic chiasm is transected before the animal is trained, the result is the same (Fig. 10-7, B). Information is presumably transferred between the two hemispheres through the corpus callosum. This finding can be confirmed by cutting both the optic chiasm and the corpus callosum before training (Fig. 10-7, C). Then the information is not transferred, and each hemisphere must learn the task independently.
|
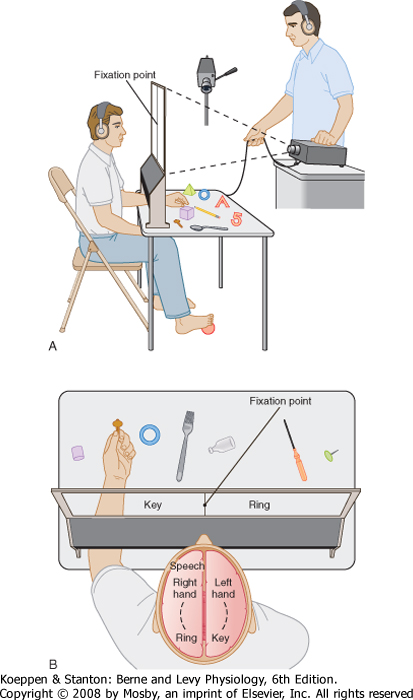
| A similar experiment has been conducted in human patients who have undergone surgical transection of the corpus callosum to prevent the interhemispheric spread of epilepsy (Fig. 10-8). The optic chiasm remained intact. Directing visual information to one or the other hemisphere was possible by having the patient fix his vision on a point on a screen. A picture of an object was then flashed to one side of the fixation point so that visual information about the picture reached only the contralateral hemisphere. An opening beneath the screen allowed the patient to manipulate objects that could not be seen. The objects included those shown in the projected pictures. Normal individuals would be able to locate the correct object with either hand. However, patients with a transected corpus callosum could locate the correct object only with the hand ipsilateral to the projected image (contralateral to the hemisphere that received the visual information). The visual information must have had access to the somatosensory and motor areas of the cortex for the hand to explore and recognize the correct object. With the corpus callosum cut, the visual and motor areas are interconnected only on the same side of the brain.
|
| Another test was to ask the patient to verbally identify what object was seen in the picture. The patient would make a correct verbal response to a picture that was projected to the right of the fixation point so that the visual information reached only the left (language dominant) hemisphere. However, the patient could not verbally identify a picture that was presented to the left hemifield so that visual information reached the right hemisphere.
|
| Similar observations can be made in patients with a transected corpus callosum when different forms of stimuli are used. For example, when such patients are given a verbal command to raise their right arm, they will do so without difficulty. The language centers in the left hemisphere send signals to the ipsilateral motor areas, and these signals produce the movement of the right arm. However, the same patients cannot respond to a command to raise their left arm. The language areas on the left side cannot influence the motor areas on the right unless the corpus callosum is intact. Somatosensory stimuli applied to the right side of the body can be described by patients with a transected corpus callosum, but these patients cannot describe the same stimuli applied to the left side of the body. Information that reaches the right somatosensory areas of the cortex cannot reach the language centers if the corpus callosum has been cut.
|
| The functional capabilities of the two hemispheres can be compared by exploring the performance of individuals with a transected corpus callosum. Such patients solve three-dimensional puzzles better with the right than with the left hemisphere, thus suggesting that the right hemisphere has specialized functions for spatial tasks. Other functions that seem to be more associated with the right than the left hemisphere are facial expression, body language, and speech intonation (Fig. 10-9). The corpus callosum promotes coordination between the two hemispheres. Patients with a transected corpus callosum lack coordination. When they are dressing, for example, one hand may button a shirt while the other tries to unbutton it. Observation of these patients indicates that the two hemispheres can operate quite independently when they are no longer interconnected. However, one hemisphere can express itself with language, whereas the other communicates only nonverbally.
|
| page 210 |  | | page 211 |
| Figure 10-7 Role of the corpus callosum in the interhemispheric transfer of visual information. A, Learning involves one eye. Discrimination depends on distinguishing between a cross and a circle. B, Discrimination is between triangles oriented with the apex up or down. C, Discrimination is between vertical and horizontal bars. |
| page 211 |  | | page 212 |
| Figure 10-8 Tests in a patient with a transected corpus callosum. A, The patient fixes on a point on a rear projection screen, and pictures are projected to either side of the fixation point. The hand can palpate objects that correspond to the projected pictures, but these objects cannot be seen. B, Response by the left hand to a picture of a key in the left field of view. However, the verbal response is that the patient sees a picture of a ring. (Redrawn from Sperry RW. In Schmitt FO, Worden FG [eds]: The Neurosciences: Third Study Program. Cambridge, MIT Press, 1974.) |
| One of the more striking examples of interhemispheric differences is the phenomenon of "cortical neglect," which is a consequence of a lesion in the parietal cortex of the nondominant, usually right hemisphere. In such cases the patient ignores objects and individuals in his visual field, draws objects that are incomplete on the left, denies that his left arm and leg are his, and fails to dress the left side of his body. He also denies that he has any such difficulties (anosognosia). Although he may respond to touch and pinprick on the left side of his body, he cannot identify objects placed in his left hand. The lesion is adjacent to SI, as well as the visual association cortex, and it suggests that this region plays a special role in the perception of one's body image and immediate extrapersonal space. Similar lesions on the dominant side result only in loss of some higher-order somesthesias, such as agraphesthesia (inability to identify characters drawn on the palm) and astereognosis (inability to identify an object only by touch). |
 |
| Major functions of the higher levels of the nervous system are learning and memory. Learning is a neural
mechanism by which the individual changes behavior as the result of experience. Memory refers to the storage mechanism for what is learned.
|
| The neural circuitry involved in memory and learning in mammals is complex; hence it is difficult to study these mechanisms. Alternative approaches are animal studies, especially in the simpler nervous systems of invertebrates, analysis of the functional consequences of lesions, and anatomic/physiological studies at the cellular and pathway level. For example, by using the marine mollusk Aplysia it has been possible to isolate a connection between a single sensory neuron and a motor neuron, which shows aspects of habituation (learning not to respond to repetitions of an insignificant stimulus), sensitization (increased responsiveness to innocuous stimuli that follow the presentation of a strong or noxious stimulus), and even associative conditioning (learning to respond to a previously insignificant event after it has been paired with a significant one). In the case of habituation, the amount of transmitter released in successive responses gradually diminishes. The change involves an alteration in the Ca++ current that triggers release of neurotransmitter. The cause of this change is inactivation of presynaptic Ca++ channels by repeated action potentials. Long-term habituation can also be produced. In this case the number of synaptic endings and active zones in the remaining terminals decreases.
|
| page 212 |  | | page 213 |
| Figure 10-9 Schematic illustration of the functional specializations of the left and right hemispheres as determined in patients after section of the corpus callosum. (Modified from Siegel A, Sapru HN: Essential Neuroscience, 5ed., Philadelphia, Lippincott Williams & Wilkins, 2005.) |
| Another model of learning is provided by a synaptic phenomenon called long-term potentiation (LTP). LTP has been studied most intensively in slices of the hippocampus in vitro. However, LTP has also been described in the neocortex and in other parts of the nervous system. Repetitive activation of an afferent pathway to the hippocampus or repetitive activation
of one of the intrinsic connections increases the responses of pyramidal cells. The increased responses (the LTP) last for hours in vitro (and even days to weeks in vivo). The forms of LTP differ, depending on the particular synaptic system. The mechanism of the enhanced synaptic efficacy seems to involve both presynaptic and postsynaptic events. The neurotransmitters involved in LTP include excitatory amino acids that act on N-methyl-d-aspartate (NMDA) receptors, the responses of which are associated with an influx of Ca++ into the postsynaptic neuron. Second messenger pathways (including G proteins, Ca++/calmodulin-dependent kinase II, protein kinase G, and protein kinase C) are also involved, and these kinases cause protein phosphorylation and changes in the responsiveness of neurotransmitter receptors. A retrograde messenger, perhaps nitric oxide (or carbon monoxide), may be released from postsynaptic neurons to act on presynaptic endings in such a way that transmitter release is enhanced. Immediate-early genes are also activated during LTP. Hence, changes in gene expression may also be involved.
|
| Another form of synaptic plasticity is long-term depression (LTD). LTD has been studied most extensively in the cerebellum, but it also occurs in the hippocampus and in other regions of the central nervous system (CNS). Some of the same factors, such as influx of Ca++ and activation of signal transduction mechanism, may account for the induction of LTD, just as for LTP.
|
| page 213 |  | | page 214 |
| With regard to the stages of memory storage, a distinction between short-term memory and long-term memory is useful. Recent events appear to be stored in short-term memory by ongoing neural activity because short-term memory persists for only minutes. Short-term memory is used, for instance, to remember
a telephone number after calling the operator. Long-term memory can be subdivided into an intermediate form, which can be disrupted, and a long-lasting form, which is difficult to disrupt. Memory loss can be caused by a disruption of memory per se, or it can be a result of interference with the mechanism for recovering information from memory. Long-term memory may involve structural changes in the nervous system because this form of memory can remain intact even after events that disrupt short-term memory.
|
| The temporal lobes appear to be particularly important for memory because bilateral removal of the hippocampal formation severely and permanently disrupts recent memory. Short-term and long-term memories are unaffected, but new long-term memories can no longer be stored. Thus, patients with such deficits remember events before their surgery but fail to recall new events, even with multiple exposure, and must be reintroduced to their therapists repeatedly. This is a loss of declarative memory involving the conscious recall of personal events, words and their meanings, and general history. Such patients, however, can still learn some tasks because they retain procedural memory, the ability to acquire problem-solving, association, and motor skills. If patients are given a complex task to perform (e.g., mirror writing), they will not only improve during the first training session but will also perform better on subsequent days despite their denial of having any experience with the task. The cerebral structures involved in procedural memory are not yet defined.
|
| Two areas important for planning and executing motor tasks are the parietal cortex and the frontal cortex, the former because it integrates sensory information needed to define the context of a task (see Chapter 7) and the latter because it has neurons that direct all the components for motor execution (see Chapter 9). Mirror neurons have been found in both the inferior parietal and the inferior frontal cortices of macaques. These cells respond during performance of a specific motor task and also during observation of the same task performed by another animal. Because these mirror cells seem to encode for and respond to very specific and particular tasks, it has been speculated that they may underlie such functions as understanding the intentions of others and empathy, as well as the ability to learn tasks from observation. In humans, EEG activity consistent with the behavior of such mirror neurons has been localized to the inferior frontal and superior parietal lobes. Autism, which involves an inability to "read" the intentions and emotions of others, has been linked to a lack of mirror neurons by such EEG evidence. |
 |
| Damage to the nervous system can induce remodeling of neural pathways and thereby alter behavior. Such
remodeling is said to reflect the plasticity of the nervous system. The CNS is much more plastic than was once believed. Plasticity is greatest in the developing brain, but some degree of plasticity remains in the adult brain as evidenced by responses to certain manipulations, such as by lesions of the brain, by sensory deprivation, or even by experience.
|
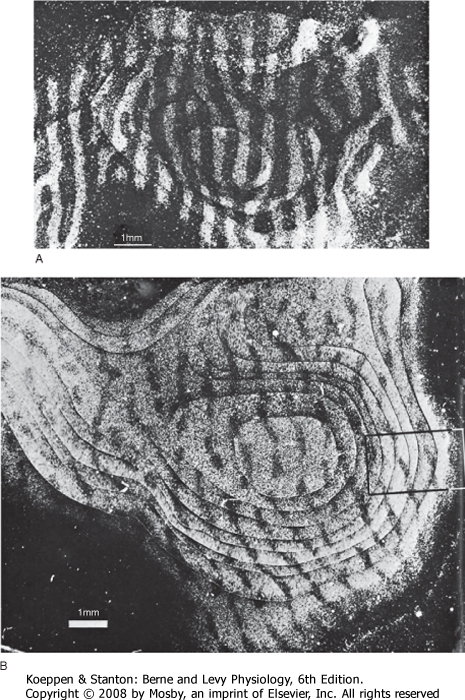
| The capability for developmental plasticity may change for some neural systems at a time referred to as the critical period. For example, it is possible to alter some connections formed in the visual pathways during their development by preventing one eye from providing input, but only during an early "critical period" in development. In these visually deprived animals, the visual connections become abnormal (Fig. 10-10), and restoration of normal visual input after this time does not undo the abnormal connections, nor does it restore functional vision from the deprived eye. In addition, similar visual deprivation for a period after several months of age does not result in abnormal connections. The plastic changes seen in such experiments may reflect a competition between fibers for synaptic connections with postsynaptic neurons in the developing nervous system. If a developing neural pathway "loses" in such a competition, the result may be a neurological deficit in the adult.
|
| Phantom limb sensation is an example of neural plasticity in adults. A patient who has suffered the amputation of a limb often perceives sensations on the missing limb when stimulated elsewhere on the body. Functional imaging studies suggest that this is a result of the spread of connections from the surviving adjacent stump into the cortical territories that had served the amputated limb.
|
| It was traditional policy to delay corrective surgery for a child born with a congenital cataract until the child was older and more able to cope with the stress of surgery. However, if the correction is deferred until after the "critical period," full recovery of function is unlikely. Similarly, children born with amblyopia, a condition characterized by strabismus (cross-eye) because of relative weakness of one of the extraocular muscles, tend to use the unaffected eye in preference. In both cases, early surgery is now common practice so that the cortical circuitry can be correctly sculpted by balanced input. |
| page 214 |  | | page 215 |
| Figure 10-10 Plasticity in the visual pathway as a result of sensory deprivation during development. The ocular dominance columns are demonstrated by autoradiography after injection of a radioactive tracer into one eye. The tracer is transported to the lateral geniculate nucleus and then transneurally transported to the striate cortex. The cortex is labeled in bands that alternate with unlabeled bands whose input is from the uninjected eye. A, Normal pattern. B, Changed pattern in an animal raised with monocular visual deprivation. The injection was made into the nondeprived eye, and the ocular dominance columns for this eye were clearly expanded. In other experiments, it could be shown that the ocular dominance columns for the deprived eye contracted. (A, From Hubel DH, Wiesel TN: Proc R Soc Lond B 198:1, 1977; B, from LeVay S et al: J Comp Neurol 191:1, 1980.) |
| page 215 |  | | page 216 |
| Figure 10-11 Representation of the digit region of the left SI cortex (A) and reorganization of this representation (B) after amputation of the second and third digits. (From Haines DE [ed]: Fundamental Neuroscience for Basic and Clinical Applications, 3rd ed. Philadelphia, Churchill Livingstone, 2006.) |
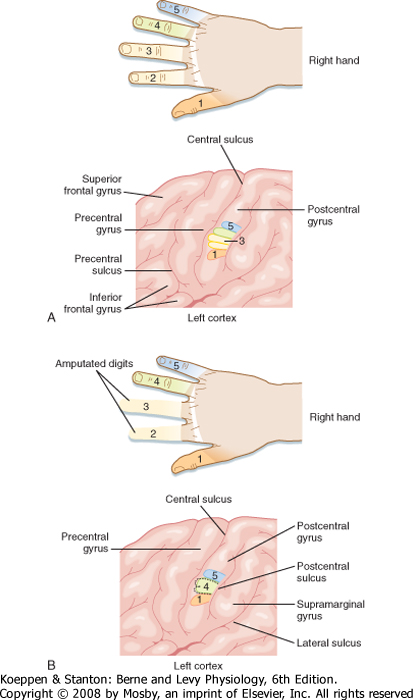
| Such remapping can also be seen after surgical amputation of the second and third digits of the hand. Before surgery, each of the digits was represented in discrete and somatotopically organized areas of the postcentral gyrus (SI). After surgery, the area that represented the amputated digits is now mapped with an enlarged representation of the adjacent digits (Fig. 10-11). Conversely, individuals born with syndactyly (i.e., fusion of two or more digits on the hand) have a single or mostly overlapping representation of these
digits in the SI cortex. After corrective surgery, the independent digits come to have distinctive representations. Even more remarkably, monkeys that were trained on a sensory discrimination task requiring repeated daily use of their fingertips showed cortical differences after training. Not only were the SI cortical territories of their fingertips larger than before training, but the number of cortically recorded receptive fields on the fingertips was likewise increased.
|
| Plastic changes can also occur after injury to the brain in adults. Sprouting of new axons does occur in the damaged CNS. However, the sprouts do not necessarily restore normal function, and many neural pathways do not appear to sprout. Additional knowledge concerning neural plasticity in the adult nervous system is vital if medical therapy is to be improved for many diseases of the nervous system and after neural trauma. Research is currently being conducted to explore the potential of human embryonic stem cells for restoring nervous system function.
|
| page 216 |  | | page 217 |
- The cerebral cortex can be subdivided into lobes based on the pattern of gyri and sulci. Each lobe has distinctive functions, as shown by the effects of lesions or seizures. The left cerebral hemisphere is dominant for language in most individuals. Wernicke's area (in the posterior temporal lobe) is responsible for the understanding of language and Broca's area (in the inferior frontal lobe) for its expression.
- The cerebral cortex can be subdivided into neocortex, allocortex, and paleocortex. The neocortex typically has six layers, whereas the other types of cortex have fewer layers. The archicortex has three layers, as typified by the hippocampus and dentate gyrus of the hippocampal formation.
- The neocortex contains a number of cell types, including pyramidal cells, which serve as the output cells, and several kinds of interneurons. The pyramidal cells release an excitatory amino acid neurotransmitter. The inhibitory interneurons are GABAergic. Specific thalamocortical afferent fibers terminate mainly in layer IV of the neocortex; diffuse thalamocortical afferent fibers synapse in layers I and VI. Cortical efferent fibers from layers II and III project to other areas of the cortex; those from layer V project to many subcortical targets, including the spinal cord, brainstem, striatum, and thalamus. Layer VI distributes to the appropriate specific thalamic nucleus.
- The cortical structure varies in different regions. Agranular cortex is found in the motor areas, whereas granular cortex occurs in the primary sensory receiving areas. Intermediate forms are found elsewhere in the neocortex. Brodmann's area designations reflect these variations in cortical structure and correlate with functionally discrete areas.
- The EEG varies with the state of the sleep-wake cycle, disease, and other factors. EEG rhythms include alpha, beta, theta, and delta waves. The EEG reflects electrical fields generated by the activity of pyramidal cells. Cortical evoked potentials are stimulus-triggered changes in the EEG and are useful clinical tests of sensory transmission. The
EEG helps in recognition of the various forms of epilepsy. Seizures are associated with depolarization shifts in pyramidal cells. Such shifts are caused by dendritic Ca++ spikes and a reduction in inhibitory processing.
- Sleep can be divided into slow-wave and REM forms. Slow-wave sleep progresses through stages 1 through 4, each with a characteristic EEG pattern. Most dreams occur in REM sleep. Sleep is produced actively by a brainstem mechanism, and its circadian rhythmicity is controlled by the suprachiasmatic nucleus.
- Information is transferred between the two hemispheres through the corpus callosum. This structure coordinates the two sides of the brain. The right hemisphere is more capable than the left in spatial tasks, facial expression, body language, and speech intonation. The left hemisphere is specialized for the understanding and generation of language and for mathematical computation.
- Learning and memory can be studied on the cellular level, in invertebrates, and in higher animals. Long-term potentiation is mediated by an increased synaptic efficacy that lasts hours to weeks and that involves both presynaptic and postsynaptic changes. Memory includes short-term (minutes), recent, and long-term storage processes and a retrieval mechanism. The hippocampal formation is important for storing declarative memory.
- Lesion studies and behavioral studies indicate that plasticity occurs in the brain throughout life. However, there appears to be more plasticity early in life, and early "critical periods" are important for the establishment of neural circuitry.
|
 |
|