| 11 The Autonomic Nervous System and Its Central Control
|
| The autonomic nervous system is often regarded as a part of the motor system. However, instead of skeletal muscle, the effectors of the autonomic nervous system are smooth muscle, cardiac muscle, and glands. Because the autonomic nervous system provides motor control of the viscera, it is sometimes called the visceral motor system. An older term for this system is the vegetative nervous system. This terminology is no longer used because it does not seem appropriate for a system that is important for all levels of activity, including aggressive behavior.
|
| By tradition, the autonomic system is a purely motor system; however, autonomic motor fibers in peripheral nerves are accompanied by visceral afferent fibers that originate from sensory receptors in the viscera. Many of these receptors trigger reflexes, but the activity of some receptors evokes sensory experiences such as pain, hunger, thirst, nausea, and a sense of visceral distention.
|
| An important function of the autonomic nervous system is to assist the body in maintaining a constant internal environment (homeostasis). When internal stimuli signal that regulation of the body's environment is required, the central nervous system (CNS) and its autonomic outflow issue commands that lead to compensatory actions. For example, a sudden increase in systemic blood pressure activates the baroreceptors, which in turn modify the activity of the autonomic nervous system so that the blood pressure is restored toward its previous level (see Chapter 17).
|
| The autonomic nervous system also participates in appropriate and coordinated responses to external stimuli. For example, the autonomic nervous system helps regulate pupil size in response to different intensities of ambient light. An extreme example of this regulation is the "fight-or-flight response" that occurs when a threat intensively activates the sympathetic nervous system. Such activation causes a variety of responses. Adrenal hormones are released, the heart rate and blood pressure increase, bronchioles dilate, intestinal motility and secretion are inhibited, glucose metabolism increases, pupils dilate, hairs become erect because of the action of piloerector muscles, cutaneous and splanchnic blood vessels constrict, and blood vessels in skeletal muscle dilate. However, the fight-or-flight response is an uncommon event; it does not represent the usual mode of operation in daily life.
|
| The term autonomic nervous system generally refers to the sympathetic and parasympathetic nervous systems. In this chapter, the enteric nervous system is also included as part of the autonomic nervous system, although it is sometimes considered a separate entity (see also Chapter 32). In addition, because the autonomic nervous system is under CNS control, the central components of the autonomic nervous system are discussed in this chapter. The central components include the hypothalamus and higher levels of the limbic system, which are associated with emotions and with many visceral types of behavior (e.g., feeding, drinking, thermoregulation, reproduction, defense, and aggression) that have survival value.
|
| ORGANIZATION OF THE AUTONOMIC NERVOUS SYSTEM
|
| The primary functional unit of the sympathetic and parasympathetic nervous systems is the two-neuron motor pathway, which consists of a preganglionic neuron, whose cell body is located in the CNS, and a postganglionic neuron, whose cell body is located in one of the autonomic ganglia (Figs. 11-1 and 11-2). The enteric nervous system includes the neurons and nerve fibers in the myenteric and submucosal plexuses, which are located in the wall of the gastrointestinal tract.
|
| page 218 |  | | page 219 |
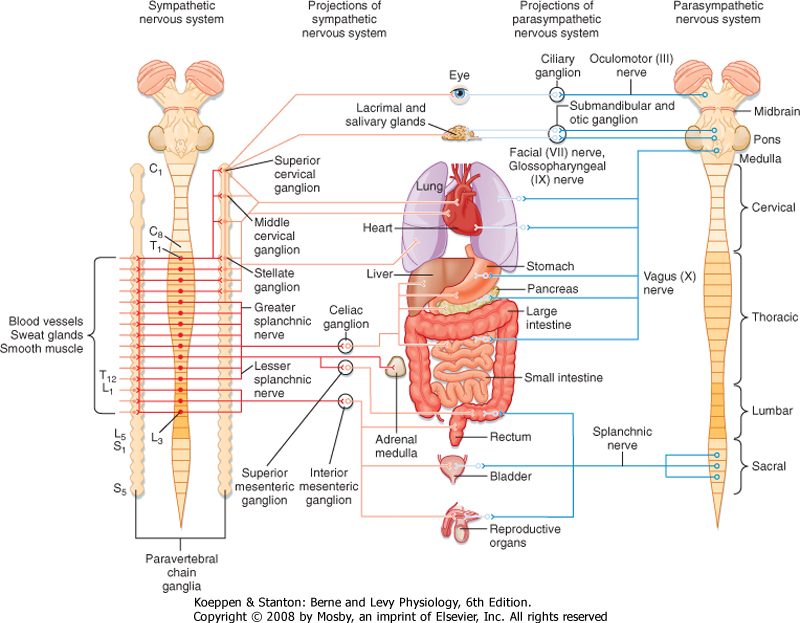
| Figure 11-1 Schematic showing the sympathetic and parasympathetic pathways. Sympathetic pathways are shown in red and parasympathetic pathways in blue. Preganglionics are shown in darker shades and postganglionics in lighter shades. |
| The sympathetic preganglionic neurons are located in the thoracic and upper lumbar segments of the spinal cord. For this reason, the sympathetic nervous system is sometimes referred to as the thoracolumbar division of the autonomic nervous system. In contrast, the parasympathetic preganglionic neurons are found in the brainstem and in the sacral spinal cord. Hence, this part of the autonomic nervous system is sometimes called the craniosacral division. Sympathetic postganglionic neurons are generally found in the paravertebral or prevertebral ganglia. The paravertebral ganglia form two sets of ganglia, one lateral to each side of the spinal cord. Each set of ganglia is linked by longitudinally running axons to form a sympathetic trunk (Figs. 11-1 and 11-2). Prevertebral ganglia are located in the abdominal cavity (Fig. 11-1).
Thus, paravertebral and prevertebral ganglia are located at some distance from their target organs. In contrast, parasympathetic postganglionic neurons are found in ganglia, which lie near or actually in the walls of the target organs.
|
| Control of the sympathetic and parasympathetic nervous systems of many organs has often been described as antagonistic. This description is not entirely correct. It is more appropriate to consider these two parts of the autonomic control system as working in a coordinated manner-sometimes acting reciprocally and sometimes synergistically-to regulate visceral function. Furthermore, not all visceral structures are innervated by both systems. For example, the smooth muscles and glands in the skin and most of the blood vessels in the body receive sympathetic innervation exclusively; only a small fraction of the blood vessels have parasympathetic innervation. The parasympathetic nervous system does not innervate the body wall, only structures in the head and in the thoracic, abdominal, and pelvic cavities.
|
| The Sympathetic Nervous System
|
| Sympathetic preganglionic neurons are concentrated in the intermediolateral cell column (lateral horn) in the thoracic and upper lumbar segments of the spinal cord (Fig. 11-2). Some neurons may also be found in the C8 segment. In addition to the intermediolateral cell column, groups of sympathetic preganglionic neurons are found in other locations, including the lateral funiculus, the intermediate region, and the part of lamina X dorsal to the central canal.
|
| page 219 |  | | page 220 |
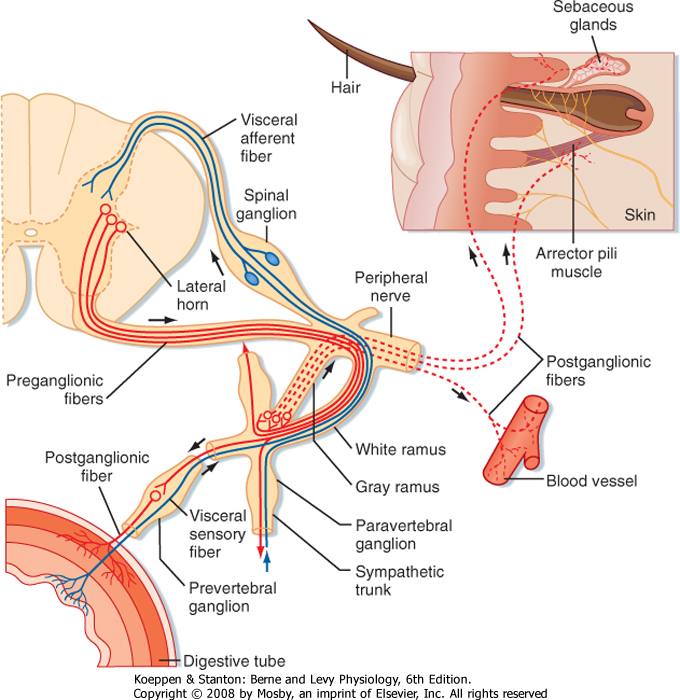
| Figure 11-2 Details of the sympathetic pathway at a spinal segment. Autonomic sensory fibers are shown in blue. Sympathetic fibers are in red with preganglionics drawn as solid lines and postganglionics as dashed lines. (Redrawn from Parent A, Carpenter MB: Carpenter's Human Neuroanatomy, 9ed., p. 295. Philadelphia, Williams & Wilkins, 1996.) |
| The axons of preganglionic neurons are often small myelinated nerve fibers known as B fibers. However, some are unmyelinated C fibers. They leave the spinal cord in the ventral root and enter the paravertebral ganglion at the same segmental level through a white communicating ramus. White rami are found only from T1 to L2. The preganglionic axon may synapse on postganglionic neurons in this ganglion; may travel rostrally or caudally within the sympathetic trunk and give off collaterals to the ganglia that it passes; or may pass through the ganglion, exit the sympathetic trunk, and enter a splanchnic nerve to travel to a prevertebral
ganglion (Figs. 11-1 and 11-2). A splanchnic nerve is a nerve that innervates the viscera; it contains both visceral afferents and autonomic fibers (sympathetic or parasympathetic).
|
| Postganglionic neurons whose somata lie in paravertebral ganglia generally send their axons through a gray communicating ramus to enter a spinal nerve (Fig. 11-2). Each of the 31 pairs of spinal nerves has a gray ramus. Postganglionic axons are distributed through the peripheral nerves to effectors, such as piloerector muscles, blood vessels, and sweat glands, located in the skin, muscle, and joints. Postganglionic axons are generally unmyelinated (C fibers), although some exceptions exist. The distinction between white and gray rami is a consequence of the relative content of myelinated and unmyelinated axons in these rami.
|
| Preganglionic axons in a splanchnic nerve often travel to a prevertebral ganglion and synapse, or they may pass through the ganglion and an autonomic plexus and end in a more distant ganglion. Some preganglionic axons pass through a splanchnic nerve and end directly on cells of the adrenal medulla, which are equivalent to postganglionic cells.
|
| The sympathetic chain extends from the cervical to the coccygeal levels of the spinal cord. This arrangement serves as a distribution system that enables preganglionic neurons, which are limited to the thoracic and upper lumbar segments, to activate postganglionic neurons that innervate all body segments. However, there are fewer paravertebral ganglia than there are spinal segments because some of the segmental ganglia fuse during development. For example, the superior cervical sympathetic ganglion represents the fused ganglia of C1 through C4, the middle cervical sympathetic ganglion is the fused ganglia of C5 and C6, and the inferior cervical sympathetic ganglion is a combination of the ganglia at C7 and C8. The term stellate ganglion refers to fusion of the inferior cervical sympathetic ganglion with the ganglion of T1. The superior cervical sympathetic ganglion provides postganglionic innervation to the head and neck, and the middle cervical and stellate ganglia innervate the heart, lungs, and bronchi.
|
| Generally, the sympathetic preganglionic neurons are distributed to ipsilateral ganglia and thus control autonomic function on the same side of the body. One important exception is that the sympathetic innervation of the intestine and the pelvic viscera is bilateral. As with motor neurons to skeletal muscle, sympathetic preganglionic neurons that control a particular organ are spread over several segments. For example, the sympathetic preganglionic neurons that control sympathetic functions in the head and neck region are distributed in C8 to T5, whereas those that control the adrenal gland are in T4 to T12.
|
| page 220 |  | | page 221 |
| The Parasympathetic Nervous System
|
| The parasympathetic preganglionic neurons are located in several cranial nerve nuclei in the brainstem, as well as in the intermediate region of the S3 and S4 segments of the sacral spinal cord (Fig. 11-1). The cranial nerve nuclei that contain parasympathetic preganglionic neurons are the Edinger-Westphal nucleus (cranial nerve III), the superior (cranial nerve VII) and inferior (cranial nerve IX) salivatory nuclei, and the dorsal motor nucleus and nucleus ambiguus (cranial nerve X). Postganglionic parasympathetic cells are located in cranial ganglia, including the ciliary ganglion (preganglionic input is from the Edinger-Westphal nucleus), the pterygopalatine and submandibular ganglia (input from the superior salivatory nucleus), and the otic ganglion (input from the inferior salivatory nucleus). The ciliary ganglion innervates the pupillary sphincter and ciliary muscles in the eye. The pterygopalatine ganglion supplies the lacrimal gland, as well as glands in the nasal and oral pharynx. The submandibular ganglion projects to the submandibular and sublingual salivary glands and to glands in the oral cavity. The otic ganglion innervates the parotid salivary gland and glands in the mouth.
|
| Other parasympathetic postganglionic neurons are located near or in the walls of visceral organs in the thoracic, abdominal, and pelvic cavities. Neurons of the enteric plexus include cells that can also be considered parasympathetic postganglionic neurons. These cells receive input from the vagus or pelvic nerves. The vagus nerves innervate the heart, lungs, bronchi, liver, pancreas, and gastrointestinal tract from the esophagus to the splenic flexure of the colon. The remainder of the colon and rectum, as well as the urinary bladder and reproductive organs, is supplied by sacral parasympathetic preganglionic neurons that travel through the pelvic nerves to postganglionic neurons in the pelvic ganglia.
|
| The parasympathetic preganglionic neurons that project to the viscera of the thorax and part of the abdomen are located in the dorsal motor nucleus of the vagus (see Fig. 4-7 E, F) and the nucleus ambiguus. The dorsal motor nucleus is largely secretomotor (it activates glands), whereas the nucleus ambiguus is visceromotor (it modifies the activity of cardiac muscle). The dorsal motor nucleus supplies visceral organs in the neck (pharynx, larynx), thoracic cavity (trachea, bronchi, lungs, heart, esophagus), and abdominal cavity (including much of the gastrointestinal tract, liver, and pancreas). Electrical stimulation of the dorsal motor nucleus results in gastric acid secretion, as well as secretion of insulin and glucagon by the pancreas. Although projections to the heart have been described, their function is uncertain. The nucleus ambiguus contains two groups of neurons: (1) a dorsal group (branchiomotor) that activates striated muscle in the soft palate, pharynx, larynx, and esophagus and (2) a ventrolateral group that innervates and slows the heart (see also Chapter 18).
|
| The visceral motor fibers in the autonomic nerves are accompanied by visceral afferent fibers. Most of these afferent fibers supply information that originates from sensory receptors in the viscera. The activity of many of these sensory receptors never reaches the level of consciousness. Instead, these receptors initiate the afferent limb of reflex arcs. Both viscerovisceral and viscerosomatic reflexes are elicited by these afferent fibers. Visceral reflexes operate at a subconscious level, and they are very important for homeostatic regulation and adjustment to external stimuli.
|
| The fast-acting neurotransmitters released by visceral afferent fibers are not well documented, although many of these neurons release an excitatory amino acid transmitter such as glutamate. However, visceral afferent fibers do contain many neuropeptides or combinations of neuropeptides, including angiotensin II, arginine vasopressin, bombesin, calcitonin gene-related peptide, cholecystokinin, galanin, substance P, enkephalin, oxytocin, somatostatin, and vasoactive intestinal polypeptide.
|
| Visceral afferent fibers that mediate sensation include nociceptors that travel in sympathetic nerves, such as the splanchnic nerves. Visceral pain is caused by excessive distention of hollow viscera, contraction against an obstruction, or ischemia. The origin of visceral pain is often difficult to identify because of its diffuse nature and its tendency to be referred to somatic structures (see Chapter 7). Visceral nociceptors in sympathetic nerves reach the spinal cord via the sympathetic chain, white rami, and dorsal roots. The terminals of nociceptive afferent fibers are distributed widely in the superficial dorsal horn and also in laminae V and X. They activate not only local interneurons, which participate in reflex arcs, but also projection cells, which include spinothalamic tract cells that signal pain to the brain.
|
| A major visceral nociceptive pathway from the pelvis involves a relay in the gray matter of the lumbosacral spinal cord. These neurons send axons into the fasciculus gracilis that terminate in the nucleus gracilis. Thus, the dorsal columns not only contain primary afferents for somatic sensation (their main component) but also second-order neurons of the visceral pain pathway (recall that second-order axons for somatic pain travel in the lateral funiculus as part of the spinothalamic tract). Visceral nociceptive signals are then transmitted to the ventral posterior lateral nucleus of the thalamus and presumably from there to the cerebral cortex. Interruption of this pathway accounts for the beneficial effects of surgically induced lesions of the dorsal column at lower thoracic levels to relieve pain produced by cancer of the pelvic organs.
|
| page 221 |  | | page 222 |
| Other visceral afferent fibers travel in parasympathetic nerves. These fibers are generally involved in reflexes rather than sensation (except for taste afferent fibers; see Chapter 8). For example, the baroreceptor afferent fibers that innervate the carotid sinus are in the glossopharyngeal nerve. They enter the brainstem,
pass through the solitary tract, and terminate in the nucleus of the solitary tract. These neurons connect with interneurons in the brainstem reticular formation. The interneurons, in turn, project to the autonomic preganglionic neurons that control heart rate and blood pressure (see Chapter 18).
|
| The nucleus of the solitary tract receives information from all visceral organs, except those in the pelvis. This nucleus is subdivided into several areas that receive information from specific visceral organs.
|
| The Enteric Nervous System
|
| The enteric nervous system, which is located in the wall of the gastrointestinal tract, contains about 100 million neurons. The enteric nervous system is subdivided into the myenteric plexus, which lies between the longitudinal and circular muscle layers of the gut, and the submucosal plexus, which lies in the submucosa of the gut. The neurons of the myenteric plexus primarily control gastrointestinal motility (see Chapter 26), whereas those in the submucosal plexus primarily regulate body fluid homeostasis (see Chapter 34).
|
| The types of neurons found in the myenteric plexus include not only excitatory and inhibitory motor neurons (which can be considered parasympathetic postganglionic neurons) but also interneurons and primary afferent neurons. Afferent neurons supply mechanoreceptors within the wall of the gastrointestinal tract. These mechanoreceptors form the afferent limb of reflex arcs within the enteric plexus. Local excitatory and inhibitory interneurons process these reflexes, and the output is sent through the motor neurons to smooth muscle cells. Excitatory motor neurons release acetylcholine and substance P; inhibitory motor neurons release dynorphin and vasoactive intestinal polypeptide. The circuitry of the enteric plexus is so extensive that it can coordinate the movements of an intestine that has been completely removed from the body. However, normal function requires innervation by the autonomic preganglionic neurons and regulation by the CNS.
|
| Activity in the enteric nervous system is modulated by the sympathetic nervous system. Sympathetic postganglionic neurons that contain norepinephrine inhibit intestinal motility, those that contain norepinephrine and neuropeptide Y regulate blood flow, and those that contain norepinephrine and somatostatin control intestinal secretion. Feedback is provided by intestinofugal neurons that project back from the myenteric plexus to the sympathetic ganglia.
|
| The submucosal plexus regulates ion and water transport across the intestinal epithelium and glandular secretion. It also communicates with the myenteric plexus to ensure coordination of the functions of the two components of the enteric nervous system. The neurons and neural circuits of the submucosal plexus are not as well understood as those of the myenteric plexus, but many of the neurons contain neuropeptides, and the neural networks are well organized.
|
| The main type of neuron in autonomic ganglia is the postganglionic neuron. These cells receive synaptic connections from preganglionic neurons, and they project to autonomic effector cells. However, many autonomic ganglia also contain interneurons. These interneurons process information within the autonomic ganglia; the enteric plexus can be regarded as an elaborate example of this kind of processing. One type of interneuron found in some autonomic ganglia contains a high concentration of catecholamines. Hence, these interneurons have been called small, intensely fluorescent (SIF) cells. SIF cells are believed to be inhibitory.
|
| Neurotransmitters in Autonomic Ganglia
|
| The classic neurotransmitter of autonomic ganglia, whether sympathetic or parasympathetic, is acetylcholine. The two classes of acetylcholine receptors in autonomic ganglia are nicotinic and muscarinic receptors, so named because of their responses to the plant alkaloids nicotine and muscarine. Nicotinic acetylcholine receptors can be blocked by such agents as curare or hexamethonium, and muscarinic receptors can be blocked by atropine. Nicotinic receptors in autonomic ganglia differ somewhat from those on skeletal muscle cells.
|
| Nicotinic and muscarinic receptors both mediate excitatory postsynaptic potentials (EPSPs), but these potentials have different time courses. Stimulation of preganglionic neurons elicits a fast EPSP, followed by a slow EPSP. The fast EPSP results from activation of nicotinic receptors, which cause ion channels to open. The slow EPSP is mediated by muscarinic receptors (primarily the M2 receptor-see Chapter 6) that inhibit the M current, a current produced by conductance of potassium.
|
| Neurons in autonomic ganglia also release neuropeptides that act as neuromodulators. Besides acetylcholine, sympathetic preganglionic neurons may release enkephalin, substance P, luteinizing hormone-releasing hormone, neurotensin, or somatostatin.
|
| Catecholamines such as norepinephrine and dopamine serve as the neurotransmitters of SIF cells in autonomic ganglia.
|
| Neurotransmitters between Postganglionic Neurons and Autonomic Effectors
|
| Sympathetic Postganglionic Neurons
|
| Sympathetic postganglionic neurons typically release norepinephrine, which excites some effector cells but inhibits others. The receptors on target cells may be either α- or β-adrenergic receptors. These receptors are further subdivided into α1, α2, β1, and β2 receptors. The distribution of these types of receptors and the actions that they mediate when activated by sympathetic postganglionic neurons are listed for various target organs in Table 11-1.
|
| page 222 |  | | page 223 |
|
Table 11-1.
Responses of Effector Organs to Autonomic Nerve Impulses |
| Effector Organs | Receptor Type | Adrenergic Impulses,1 Responses2 | Cholinergic Impulses,1Responses2 |
| Eye |
| Radial muscle, iris | α | Contraction (mydriasis) ++ | - |
| Sphincter muscle, iris | α | - | Contraction (miosis) +++ |
| Ciliary muscle | β | Relaxation for far vision + | Contraction for near vision +++ |
| Heart |
| Sinoatrial node | β1 | Increase in heart rate ++ | Decrease in heart rate; vagal arrest +++ |
| Atria | β1 | Increase in contractility and conduction velocity ++ | Decrease in contractility and (usually) increase in conduction velocity ++ |
| Atrioventricular node | β1 | Increase in automaticity and conduction velocity ++ | Decrease in conduction velocity; AV block +++ |
| His-Purkinje system | β1 | Increase in automaticity and conduction velocity +++ | Little effect |
| Ventricles | β1 | Increase in contractility, conduction velocity, automaticity, and rate of idioventricular pacemakers +++ | Slight decrease in contractility |
| Arterioles |
| Coronary | α, β2 | Constriction +; dilation3 ++ | Dilation + |
| Skin and mucosa | α | Constriction +++ | Dilation4 |
| Skeletal muscle | α, β2 | Constriction ++; dilation3,5 ++ | Dilation6 + |
| Cerebral | α | Constriction (slight) | Dilation4 |
| Pulmonary | α, β2 | Constriction +; dilation3 | Dilation4 |
| Abdominal viscera, renal | α, β2 | Constriction +++; dilation5+ | - |
| Salivary glands | α | Constriction +++ | Dilation ++ |
| Veins (systemic) | α, β2 | Constriction ++; dilation ++ | - |
| Lung |
| Bronchial muscle | β2 | Relaxation + | Contraction ++ |
| Bronchial glands | ? | Inhibition (?) | Stimulation +++ |
| Stomach |
| Motility and tone | α2, β2 | Decrease (usually)7 + | Increase +++ |
| Sphincters | α | Contraction (usually) + | Relaxation (usually) + |
| Secretion | | Inhibition (?) | Stimulation +++ |
| Intestine |
| Motility and tone | α2, β2 | Decrease7+ | Increase +++ |
| Sphincters | α | Contraction (usually) + | Relaxation (usually) + |
| Secretion | | Inhibition (?) | Stimulation +++ |
| Gallbladder and ducts | | Relaxation + | Contraction + |
| Kidney | β2 | Renin secretion ++ | - |
| Urinary bladder |
| Detrusor | β | Relaxation (usually) + | Contraction +++ |
| Trigone and sphincter | α | Contraction +++ | Relaxation ++ |
| Ureter |
| Motility and tone | α | Increase (usually) | Increase (?) |
| Uterus | α, β2 | Pregnant: contraction (α); nonpregnant: relaxation (β) | Variable8 |
| Sex organs, male | α | Ejaculation +++ | Erection +++ |
| Skin |
| Pilomotor muscles | α | Contraction ++ | - |
| Sweat glands | α | Localized secretion9 + | Generalized secretion +++ |
| Spleen capsule | α, β2 | Contraction +++; relaxation + | - |
| Adrenal medulla | | - | Secretion of epinephrine and norepinephrine |
| Liver | α, β2 | Glycogenolysis, gluconeogenesis10 +++ | Glycogen synthesis + |
| Pancreas |
| Acini | α | Decreased secretion + | Secretion ++ |
| Islets (beta cells) | α | Decreased secretion +++ | - |
| | β2 | Increased secretion + | - |
| Fat cells | α, β1 | Lipolysis10+++ | - |
| Salivary glands | α | K+ and water secretion + | K+ and water secretion +++ |
| | β | Amylase secretion + | - |
| Lacrimal glands | | - | Secretion +++ |
| Nasopharyngeal glands | | - | Secretion +++ |
| Pineal gland | β | Melatonin synthesis | - |
1A long dash (-) signifies no known functional innervation.
2Responses are designated + to +++ to provide an approximate indication of the importance of adrenergic and cholinergic nerve activity in control of the various organs and functions listed.
3Dilation predominates in situ because of metabolic autoregulatory phenomena.
4Cholinergic vasodilation at these sites is of questionable physiological significance.
5Over the usual concentration range of physiologically released, circulating epinephrine, a β receptor response (vasodilation) predominates in blood vessels of skeletal muscle and the liver and an α receptor response (vasoconstriction) in blood vessels of other abdominal viscera. The renal and mesenteric vessels also contain specific dopaminergic receptors, activation of which causes dilation, but their physiological significance has not been established.
6 The sympathetic cholinergic system causes vasodilation in skeletal muscle, but this is not involved in most physiological responses.
7It has been proposed that adrenergic fibers terminate at inhibitory β receptors on smooth muscle fibers and at inhibitory αreceptors on parasympathetic cholinergic (excitatory) ganglion cells of Auerbach's plexus.
8 Depends on the stage of the menstrual cycle, the amount of circulating estrogen and progesterone, and other factors.
9 Palms of the hands and some other sites ("adrenergic sweating").
10 There is significant variation among species in the type of receptor that mediates certain metabolic responses. From Goodman LS, Gilman A: The Pharmacological Basis of Therapeutics, 6th ed. New York, Macmillan, 1980.
|
| α1 Receptors are located postsynaptically, but α2 receptors may be either presynaptic or postsynaptic. Receptors located presynaptically are generally called autoreceptors; they usually inhibit release of transmitter. The effects of agents that excite α1 or α2 receptors can be distinguished by using antagonists to block these receptors specifically. For example, prazosin is a selective α1-adrenergic antagonist, and yohimbine is a selective α2-adrenergic antagonist. The effects of α1 receptors are mediated by activation of the inositol triphosphate/diacylglycerol second messenger system (see Chapter 3). In contrast, α2 receptors decrease the rate of synthesis of cAMP through action on a G protein.
|
| β Receptors are subdivided into β1 and β2 receptors on the basis of the ability of antagonists to block them. The proteins that make up the two types of β receptors are similar, with seven membrane-spanning regions connected by intracellular and extracellular domains (see Chapter 3). Agonist drugs that work on β receptors activate a G protein that stimulates adenylyl cyclase to increase the cAMP concentration. This action is terminated by the buildup of guanosine diphosphate.
|
| β Receptors can also be antagonized by the action of α1 receptors. The number of β receptors can be regulated. If the β receptors are exposed to agonists, they can be desensitized by phosphorylation. In addition, their numbers can be decreased if they become internalized. β Receptors can also increase in number (up-regulation), for example, by denervation. The number of α receptors is likewise regulated.
|
| In addition to releasing norepinephrine, sympathetic postganglionic neurons release neuropeptides such as somatostatin and neuropeptide Y. For example, cells that release both norepinephrine and somatostatin supply the mucosa of the gastrointestinal tract, and cells that release both norepinephrine and neuropeptide Y innervate blood vessels in the gut and the limb. Another chemical mediator in sympathetic postganglionic neurons is ATP.
|
| The endocrine cells of the adrenal medulla are similar in many respects to sympathetic postganglionic neurons (see also Chapter 42). They receive input from sympathetic preganglionic neurons, are excited by acetylcholine, and release catecholamines. However, the cells of the adrenal medulla differ from sympathetic postganglionic neurons in that they release catecholamines into the circulation rather than into a synapse. Moreover, the main catecholamine released is epinephrine, not norepinephrine. In humans, 80% of the catecholamine released by the adrenal medulla is epinephrine and 20% is norepinephrine.
|
| Some sympathetic postganglionic neurons release acetylcholine rather than norepinephrine as their neurotransmitter. For example, sympathetic postganglionic neurons that innervate eccrine sweat glands are cholinergic. The acetylcholine receptors involved are muscarinic, and they are therefore blocked by atropine. Similarly, some blood vessels are innervated by cholinergic sympathetic postganglionic neurons. In addition to releasing acetylcholine, the postganglionic neurons that supply the sweat glands also release neuropeptides, including calcitonin gene-related peptide and vasoactive intestinal polypeptide.
|
| Parasympathetic Postganglionic Neurons
|
| page 224 |  | | page 225 |
| The neurotransmitter released by parasympathetic postganglionic neurons is acetylcholine. The effects of these neurons on various target organs are listed in Table 11-1. Parasympathetic postganglionic actions are mediated by muscarinic receptors. On the basis of binding studies, the action of selective antagonists, and molecular cloning, five types of muscarinic receptors
have now been discovered (see Chapter 6). Activation of M1 receptors enhances the secretion of gastric acid in the stomach. The M2 receptor is the most abundant receptor type in smooth muscle, including smooth muscle in the intestines, uterus, trachea, and bladder. In addition, it is found in autonomic ganglia and in the heart, where they exert negative chronotropic and inotropic actions (see Chapter 18). M3 receptors are also found in the smooth muscle of a variety of organs, and although they are less abundant than M2 receptors, normal contractile patterns appear to require an interaction between the two receptor types. M4 receptors, like M2 receptors, are found in autonomic ganglia and thus play a role in synaptic transmission at these sites. M5 receptors are found in the sphincter muscle of the pupil, the esophagus, and the parotid gland, as well as in cerebral blood vessels.
|
| Chagas' disease is the result of infection by the parasite Trypanosoma cruzi. About 18 million people are infected worldwide, and approximately 50,000 die each year as a result of complications from the disease. The most serious forms involve enlargement of the esophagus, colon, and heart. Loss of parasympathetic control is a significant component of the initial stages of the disease; shortly after the initial infection, the parasympathetic neurons innervating the heart, esophagus, and colon are destroyed, which leads to arrhythmias (and potentially sudden death) and aperistalsis. Chronically, cardiomyopathy (malfunction of the heart muscle) that can lead to death occurs in approximately 30% of those infected. Although the pathogenesis of the cardiomyopathy is not fully understood, one leading idea involves autoimmunity. Antibodies against the parasitic antigens have been found to bind to the β-adrenergic and M2 acetylcholine receptors in the heart. These antibodies not only trigger autoimmune responses that destroy heart muscle but also act as agonists at these receptors and cause inappropriate responses of the cardiovascular system to changing external demands. |
 |
| Muscarinic receptors, like adrenergic receptors, have diverse actions. Some of their effects are mediated by specific second messenger systems. For example, cardiac M2 muscarinic receptors may act by way of the inositol triphosphate system, and they may also inhibit adenylyl cyclase and thus cAMP synthesis. Muscarinic receptors also open or close ion channels, particularly K+ or Ca++ channels. This action on ion channels is likely to occur through activation of G proteins. A third action of muscarinic receptors is to relax vascular smooth muscle by an effect on endothelial cells, which produce endothelium-derived relaxing factor (EDRF). EDRF is actually nitric oxide, a gas released when arginine is converted to citrulline by nitric oxide synthase (see Chapter 18). Nitric oxide
relaxes vascular smooth muscle by stimulating guanylate cyclase and thereby increasing levels of cGMP, which in turn activates a cGMP-dependent protein kinase (see Chapter 3).
|
| The number of muscarinic receptors is regulated, and exposure to muscarinic agonists decreases the number of receptors by internalization of the receptors.
|
| CENTRAL CONTROL OF AUTONOMIC FUNCTION
|
| The discharges of autonomic preganglionic neurons are controlled by pathways that synapse on autonomic preganglionic neurons. The pathways that influence autonomic activity include spinal cord and brainstem reflex pathways, as well as descending control systems originating at higher levels of the nervous system, such as the hypothalamus.
|
| Examples of Autonomic Control of Particular Organs
|
| The autonomic control of different target organs depends on local reflex circuitry and on signals from parts of the CNS (Table 11-1).
|
| The sphincter and dilator muscles of the iris determine the size of the pupil. Activation of sympathetic innervation of the eye dilates the pupil, which occurs during emotional excitement and also in response to painful stimulation. The neurotransmitter at the sympathetic postganglionic synapses is norepinephrine, and it acts at α receptors.
|
| Sympathetic control of the pupil is sometimes affected by disease. For example, interruption of the sympathetic innervation of the head and neck results in Horner's syndrome. This syndrome is characterized by the triad of miosis (abnormal pupillary constriction), ptosis (caused by paralysis of the superior tarsal muscle), and anhidrosis (loss of sweating) on the face. Enophthalmos (retraction of the eye into the orbit) also occurs in some animals (rats, cats, and dogs, among others), but in humans no true enophthalmos occurs; however, there is an apparent enophthalmos, an illusion created by partial closure of the eyelid from the ptosis. Horner's syndrome can be produced by a lesion that (1) destroys the sympathetic preganglionic neurons in the upper thoracic spinal cord, (2) interrupts the cervical sympathetic chain, or (3) damages the lower brainstem in the region of the reticular formation, through which pathways descend to the spinal cord to activate sympathetic preganglionic neurons. |
 |
| page 225 |  | | page 226 |
| The parasympathetic nervous system exerts an action on pupillary size opposite that of the sympathetic
nervous system. Whereas the sympathetic system elicits pupillary dilation, the parasympathetic system constricts the pupil. The main neurotransmitter at the postganglionic parasympathetic synapse is acetylcholine, which acts on muscarinic receptors. However, neuromodulatory peptides may also be released from some neurons.
|
| Pupil size is reduced by the pupillary light reflex and during accommodation for near vision. In the pupillary light reflex, light that strikes the retina is processed by retinal circuits that excite W-type retinal ganglion cells (see Chapter 8). These cells respond to diffuse illumination. The axons of some of the W cells project through the optic nerve and tract to the pretectal area, where they synapse in the olivary pretectal nucleus. This nucleus contains neurons that also respond to diffuse illumination. Activity of neurons of the olivary pretectal nucleus causes pupillary constriction by means of bilateral connections with parasympathetic preganglionic neurons in the Edinger-Westphal nuclei. The reflex results in contraction of the pupillary sphincter muscles in both eyes.
|
| The pupillary light reflex is sometimes absent in patients with syphilis, which affects the CNS (i.e., in tabes dorsalis). Although the pupil fails to respond to light, it has a normal accommodation response. This condition is known as the Argyll Robertson pupil. The exact mechanism is controversial. One explanation rests on the fact that some optic tract fibers project to the pretectal area in the midbrain. These fibers can be damaged in syphilitic meningitis, possibly by the presence of spirochetes in the subarachnoid space. Note that the pretectal area projects to the Edinger-Westphal nucleus, also in the midbrain, whose cells originate the parasympathetic innervation of the eye, which controls the pupillary sphincter muscle. Although input to the olivary pretectal nucleus is interrupted, the optic tract fibers projecting to the lateral geniculate nucleus are not destroyed, and thus vision is maintained, as is pupillary constriction during accommodation. |
 |
| In the accommodation response, information from M cells of the retina is transmitted to the striate cortex through the geniculostriate visual pathway (see Chapter 8). The stimulus that triggers accommodation is thought to be a blurred retinal image and disparity of the image between the two eyes. After the information is processed in the visual cortex, signals are transmitted directly or indirectly to the middle temporal cortex, where they activate neurons in a visual area known as MT. MT neurons transmit signals to the midbrain that activate parasympathetic preganglionic neurons in the Edinger-Westphal nuclei bilaterally via their axons in cranial nerve III, which results in pupillary constriction. At the same time signals are transmitted to the ciliary muscle that cause it to contract. This ciliary muscle contraction allows the lens to round up and increase its refractile power.
|
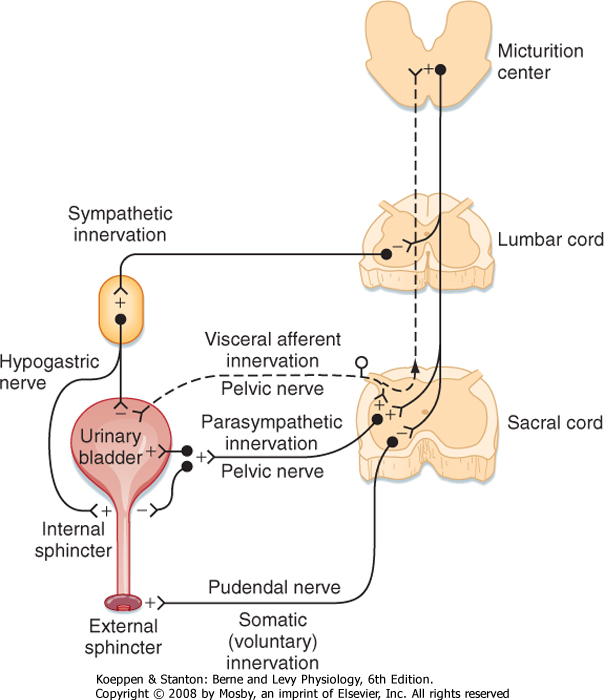
| The urinary bladder is controlled by reflex pathways in the spinal cord and also by a supraspinal center (Fig. 11-3). The sympathetic innervation originates from preganglionic sympathetic neurons in the upper lumbar segments of the spinal cord. Postganglionic sympathetic axons act to inhibit the smooth muscle (detrusor muscle) throughout the body of the bladder, and they also act to excite the smooth muscle of the trigone region and the internal urethral sphincter. The detrusor muscle is tonically inhibited during filling of the bladder, and such inhibition prevents urine from being voided. Inhibition of the detrusor muscle is mediated by the action of norepinephrine on β receptors, whereas excitation of the trigone and internal urethral sphincter is elicited by the action of norepinephrine on α receptors.
|
| The external sphincter of the urethra also helps prevent voiding. This sphincter is a striated muscle, and it is innervated by motor axons in the pudendal nerves, which are somatic nerves. The motor neurons are located in Onuf's nucleus, in the ventral horn of the sacral spinal cord.
|
| The parasympathetic preganglionic neurons that control the bladder are located in the sacral spinal cord (the S2 and S3 or S3 and S4 segments). These cholinergic neurons project through the pelvic nerves and are distributed to ganglia in the pelvic plexus and the bladder wall. Postganglionic parasympathetic neurons in the bladder wall innervate the detrusor muscle, as well as the trigone and sphincter. The parasympathetic activity contracts the detrusor muscle and relaxes the trigone and sphincter. These actions result in micturition, or urination. Some of the postganglionic neurons are cholinergic and others are purinergic (they release ATP).
|
| Micturition is normally controlled by the micturition reflex (see Fig. 11-3). Mechanoreceptors in the bladder wall are excited by both stretch and contraction of the muscles in the bladder wall. Thus, as urine accumulates and distends the bladder, the mechanoreceptors begin to discharge. The pressure in the urinary bladder is low during filling (5 to 10 cm H2O), but it increases abruptly when micturition begins. Micturition can be triggered either reflexively or voluntarily. In reflex micturition, bladder afferent fibers excite neurons that project to the brainstem and activate the micturition center in the rostral pons (Barrington's center). The ascending projections also inhibit sympathetic preganglionic neurons that prevent voiding. When a sufficient level of activity occurs in this ascending pathway, micturition is triggered by the micturition center. Commands reach the sacral spinal cord through a reticulospinal pathway. Activity in the sympathetic projection to the bladder is inhibited, and the parasympathetic projections to the bladder are activated. Contraction of muscle in the wall of the bladder causes a vigorous discharge of the mechanoreceptors that supply the bladder wall and thereby further activates the supraspinal loop. The result is complete emptying of the bladder.
|
| page 226 |  | | page 227 |
| Figure 11-3 Descending and efferent pathways for reflexes that control the urinary bladder. The ascending and afferent parts of reflex arcs are not drawn, but see text for their description. (Redrawn from de Groat WC, Booth AM. In Dyck PJ et al (eds): Peripheral Neuropathy, 2nd ed. Philadelphia, WB Saunders, 1984.) |
| A spinal reflex pathway also exists for micturition. This pathway is operational in newborn infants. However, with maturation, the supraspinal control pathways take on a dominant role in triggering micturition. After spinal cord injury, human adults lose bladder control during the period of spinal shock (urinary incontinence). As the spinal cord recovers from spinal shock, some degree of bladder function is recovered because of enhancement of the spinal cord micturition reflex. However, the bladder has increased muscle tone and fails to empty completely. These circumstances frequently lead to urinary infections.
|
| Autonomic Centers in the Brain
|
| An autonomic center consists of a local network of neurons that respond to input from a particular source and that influence distant neurons by way of long efferent pathways. For example, the micturition center is the autonomic center in the pons that regulates micturition. Many other autonomic centers with diverse functions are also located in the brain. Vasomotor and vasodilator centers are in the medulla, and respiratory centers are in the medulla and pons. Perhaps the greatest concentration of autonomic centers is found in the hypothalamus.
|
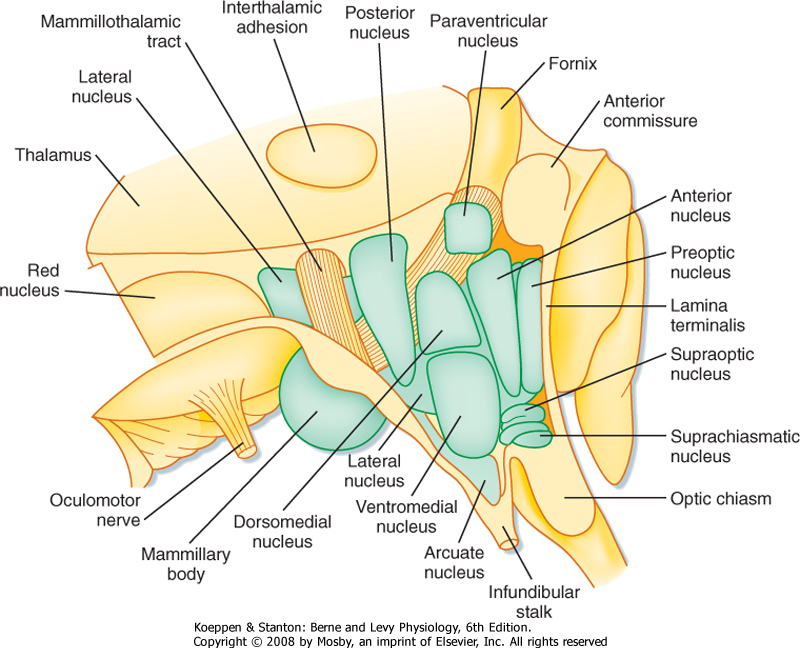
| Figure 11-4 Main nuclei of the hypothalamus seen in a view from the third ventricle. Anterior is to the right. (Redrawn from Nauta WJH, Haymaker W: The Hypothalamus. Springfield, IL, Charles C Thomas, 1969.) |
| page 227 |  | | page 228 |
| The hypothalamus is part of the diencephalon. Some of the nuclei of the hypothalamus are shown in Figure 11-4. In the rostrocaudal dimension, the hypothalamus can be subdivided into three regions: suprachiasmatic, tuberal, and mammillary regions. Continuing anteriorly from the hypothalamus are telencephalic structures, the preoptic region and septum. Both the preoptic and septal regions help regulate autonomic
function. Important fiber tracts that course through the hypothalamus are the fornix, the medial forebrain bundle, and the mammillothalamic tract. The fornix is used as a landmark to divide the hypothalamus into the medial and lateral hypothalamus.
|
| The hypothalamus has many functions; see Chapter 40 for a discussion of hypothalamic control of endocrine function. Its control of autonomic function is emphasized here.
|
| Homeothermic animals are those that are able to regulate their body temperature. When the environmental temperature decreases, the body adjusts by reducing heat loss and by increasing heat production. Conversely, when the temperature rises, the body increases its heat loss and reduces heat production.
|
| Information about the external temperature is provided by thermoreceptors in the skin (and probably other organs such as muscle). Internal temperature is monitored by central thermoreceptive neurons in the anterior hypothalamus. The central thermoreceptors monitor the temperature of blood. The system acts as a servomechanism (a control system that uses negative feedback to operate another system) with a set point at the normal body temperature. Error signals, which represent a deviation from the set point, evoke responses that tend to restore body temperature toward the set point. These responses are mediated by the autonomic, somatic, and endocrine systems.
|
| Cooling causes shivering, which consists of asynchronous muscle contractions that increase heat production. Increases in thyroid gland activity and in sympathetic neural activity tend to increase heat production metabolically. Heat loss is reduced by piloerection and by cutaneous vasoconstriction. Piloerection is effective in animals with fur but not in humans; in the latter, the result is goose bumps. In addition, the hypothalamus, via its widespread connections to cortical regions, will influence the decision to initiate concurrent somatic behavior, in this case possibly putting a jacket on.
|
| Warming the body causes changes in the opposite direction. The activity of the thyroid gland diminishes, which leads to reduced metabolic activity and less heat production. Heat loss is increased by sweating and cutaneous vasodilation.
|
| In fever, the set point for body temperature is elevated. This can be caused by the release of a pyrogen by microorganisms. The pyrogen changes the set point, thereby leading to increased heat production by shivering and to heat conservation by cutaneous vasoconstriction. |
 |
| The hypothalamus serves as the temperature servomechanism. The heat loss responses are organized by the heat loss center, which is composed of neurons in the preoptic region and anterior hypothalamus. As might be expected, lesions here prevent sweating and cutaneous vasodilation, and if the individual is placed in a warm environment, hyperthermia will occur. Conversely, electrical stimulation of the heat loss center causes cutaneous vasodilation and inhibits shivering. Heat conservation responses are organized in the posterior hypothalamus by neurons that form a heat production and conservation center. Thus, lesions in the area dorsolateral to the mammillary body interfere with heat production and conservation and can cause hypothermia when the subject is in a cold environment.
Electrical stimulation in this region of the brain evokes shivering.
|
| Thermoregulatory responses are also produced when the hypothalamus is locally warmed or cooled. These responses reflect the presence of central thermoreceptive neurons in the hypothalamus.
|
| Regulation of Food Intake
|
| Food intake is also regulated by a servomechanism. However, the set point is affected by many factors. Sensory signals that help regulate food intake operate both on a short-term basis to control ingestion and on a long-term basis to control body weight. Glucoreceptors in the hypothalamus sense blood glucose and use this information to control food intake. Their main action occurs when blood glucose levels decrease. Opioid peptides and pancreatic polypeptide stimulate food intake; cholecystokinin inhibits food intake. Insulin and adrenal glucocorticoids also affect food intake (see Chapters 38 and 42).
|
| Lesions of the lateral hypothalamus suppress food intake (aphagia), which can cause starvation and death. Electrical excitation of the lateral hypothalamus stimulates eating. These observations suggest that the lateral hypothalamus contains a feeding center. Converse effects are produced by manipulation of the ventromedial nucleus of the hypothalamus. A lesion here causes hyperphagia, which is an increased food intake that can result in obesity, whereas electrical stimulation of the same region stops the feeding behavior. This area of the hypothalamus is known as the satiety center. The feeding and satiety centers operate reciprocally.
|
| Further work is needed to clarify the role of other parts of the nervous system in feeding behavior.
|
| Regulation of Water Intake
|
| Water intake also depends on a servomechanism. Fluid intake is influenced by blood osmolality and volume (Fig. 11-5).
|
| page 228 |  | | page 229 |
| Figure 11-5 A, Structures thought to play a role in the regulation of water intake in rats. B, Neural circuits that signal changes in blood osmolality and volume. (A, Redrawn from Shepherd GM: Neurobiology. New York, Oxford University Press, 1983.) |
| With water deprivation, the extracellular fluid becomes hyperosmotic, which in turn causes the intracellular fluid to become hyperosmotic. The brain contains neurons that serve as osmoreceptors for detection of increases in the osmotic pressure of extracellular fluid (see also Chapter 34). The osmoreceptors appear to be located in the organum vasculosum of the lamina terminalis, which is a circumventricular organ. Circumventricular organs surround the cerebral ventricles and lack a blood-brain barrier. The subfornical organ and the organum vasculosum are
involved in thirst. The area postrema serves as a chemosensitive zone that triggers vomiting.
|
| Water deprivation also causes a decrease in blood volume, which is sensed by receptors in the low-pressure side of the vasculature, including the right atrium (see also Chapter 17). In addition, decreased blood volume triggers the release of renin by the kidney. Renin breaks down angiotensinogen into angiotensin I, which is then hydrolyzed to angiotensin II (see Chapter 34). This peptide stimulates drinking by an action on angiotensin II receptors in another one of the circumventricular organs, namely, the subfornical organ. Angiotensin II also causes vasoconstriction and release of aldosterone and antidiuretic hormone (ADH).
|
| Insufficient water intake is usually a greater problem than excess water intake. When more water is taken in than required, it is easily eliminated by inhibition of the release of ADH from neurons in the supraoptic nucleus at their terminals in the posterior pituitary gland (see Chapter 40). As mentioned previously, signals that inhibit release of ADH include increased blood volume and decreased osmolality of extracellular fluid. Other areas of the hypothalamus, particularly the preoptic region and lateral hypothalamus, help regulate water intake, as do several structures outside the hypothalamus.
|
| Other Autonomic Control Structures
|
| Several regions of the forebrain other than the hypothalamus also play a role in autonomic control. These regions include the central nucleus of the amygdala and the bed nucleus of the stria terminalis, as well as a number of areas of the cerebral cortex. Information reaches these higher autonomic centers from viscera through an ascending system that involves the nucleus of the solitary tract, the parabrachial nucleus, the periaqueductal gray matter, and the hypothalamus. Descending pathways that help control autonomic activity originate in such structures as the paraventricular nucleus of the hypothalamus, the A5 noradrenergic cell group, the rostral ventrolateral medulla, and the raphe nuclei and adjacent structures of the ventromedial medulla.
|
| Neural Influences on the Immune System
|
| Environmental stress can cause immunosuppression, in which the number of helper T cells and the activity of natural killer cells are reduced. Immunosuppression can even be the result of classic conditioning. One mechanism for such an effect involves the release of corticotropin-releasing factor (CRF) from the hypothalamus. CRF causes the release of adrenocorticotropic hormone (ACTH) from the pituitary gland; release of ACTH stimulates the secretion of adrenal corticosteroids, which cause immunosuppression (see Chapter 42). Other mechanisms include direct neural actions on lymphoid tissue. The immune system may also influence neural activity.
|
| The limbic system helps control emotional behavior, in part by an influence on the hypothalamus. The limbic lobe is phylogenetically the oldest part of the cerebral cortex. A circuit that connects the limbic lobe with the hypothalamus (the Papez circuit) regulates emotional behavior. The neural components of this circuit are termed the limbic system (Fig. 11-6, see also Fig. 10-1).
|
| The Papez circuit connects many areas of the neocortex to the hypothalamus. Information passes from the cingulate gyrus to the entorhinal cortex and hippocampus and from there via the fornix to the mammillary bodies in the hypothalamus. The mammillothalamic tract then connects the hypothalamus with the anterior thalamic nuclei, which project back to the cingulate gyrus. Other structures included in the limbic system circuitry are the amygdala and the bed nucleus of the stria terminalis.
|
| page 229 |  | | page 230 |
| Figure 11-6 The Papez circuit. (From Groves PM, Schlesinger K: Introduction to Biological Psychology, 2nd ed. Dubuque, IA, William C Brown, 1982.) |
| Bilateral temporal lobe lesions can produce Klüver-Bucy syndrome, which is characterized by loss of the ability to detect and recognize the meaning of objects from visual cues (visual agnosia), a tendency to examine objects orally, attention to irrelevant stimuli, hypersexuality, change in dietary habits, and decreased emotionality. The components of this syndrome can be attributed to damage to different parts of the neocortex and limbic cortex. For instance, changes in emotional behavior are largely the result of lesions of the amygdala, whereas visual agnosia is caused by damage to visual areas in the temporal neocortex.
|
- The autonomic nervous system is a motor system that controls smooth muscle, cardiac muscle, and glands. It helps maintain homeostasis and coordinates responses to external stimuli. Its components are the sympathetic, parasympathetic, and enteric nervous systems. Autonomic motor pathways have preganglionic and postganglionic neurons. Preganglionic neurons reside in the CNS, whereas postganglionic neurons lie in peripheral ganglia.
- Sympathetic preganglionic neurons are located in the thoracolumbar region of the spinal cord, and sympathetic postganglionic neurons are located in paravertebral and prevertebral ganglia. Parasympathetic preganglionic neurons are located in cranial nerve nuclei or in the sacral spinal cord. Parasympathetic postganglionic neurons reside in ganglia located in or near the target organs.
- Visceral afferent fibers innervate sensory receptors in the viscera. Most function to activate reflexes, but some also have a sensory function, such as visceral pain and taste.
- The enteric nervous system includes the myenteric and submucosal plexuses in the wall of the gastrointestinal tract. The myenteric plexus regulates motility, and the submucosal plexus regulates ion and water transport and secretion.
- Neurotransmitters at the synapses of preganglionic neurons in autonomic ganglia include acetylcholine (acting at both nicotinic and muscarinic receptors) and a number of neuropeptides. Interneurons in the ganglia release catecholamines. Sympathetic postganglionic neurons generally release norepinephrine (acting on adrenergic receptors) as their neurotransmitter, although neuropeptides are also released. Sympathetic postganglionic neurons that supply sweat glands release acetylcholine. Parasympathetic postganglionic neurons release acetylcholine (acting on muscarinic receptors).
- The pupil is controlled reciprocally by the sympathetic and parasympathetic nervous systems. Sympathetic activity causes pupillary dilation (mydriasis); parasympathetic activity causes pupillary constriction (meiosis).
- Emptying of the urinary bladder depends on parasympathetic outflow during the micturition reflex. Sympathetic constriction of the internal sphincter of the urethra prevents voiding. The micturition reflex is triggered by stretch receptors, and it is controlled in normal adults by a micturition center in the pons.
- The hypothalamus contains several centers that control autonomic and other activities, including heat loss, heat production and conservation, feeding and satiety, and fluid intake.
- The limbic system consists of several cortical and subcortical structures. It controls emotional behavior, in part by activation of the autonomic nervous system.
|
 |
|