| 26 Functional Anatomy and General Principles of Regulation in the Gastrointestinal Tract
|
| The gastrointestinal (GI) tract consists of the alimentary tract from the mouth to the anus and includes the associated glandular organs that empty their contents into the tract. The overall function of the GI tract is to absorb nutrients and water into the circulation and eliminate waste products. The major physiological processes that occur in the GI tract are motility, secretion, digestion, and absorption. Most of the nutrients in the diet of mammals are taken in as solids and as macromolecules that are not readily transported across cell membranes to enter the circulation. Thus, digestion consists of physical and chemical modification of food such that absorption can occur across intestinal epithelial cells. Digestion and absorption require motility of the muscular wall of the GI tract to move the contents along the tract and to mix the food with secretions. Secretions from the GI tract and associated organs consist of enzymes, biological detergents, and ions that provide an intraluminal environment optimized for digestion and absorption. These physiological processes are highly regulated to maximize digestion and absorption, and the GI tract is endowed with complex regulatory systems to ensure that this occurs. In addition, the GI tract absorbs drugs administered by the oral or rectal routes.
|
| The GI tract also serves as an important organ for the excretion of substances. The GI tract stores and excretes waste substances from ingested food materials and excretes products from the liver such as cholesterol, steroids, and drug metabolites (all sharing the common property of being lipid-soluble molecules).
|
| When considering the physiology of the GI tract it is important to remember that it is a long tube that is in contact with the body's external environment. As such, it is vulnerable to infectious microorganisms that can enter along with food and water. To protect itself, the GI tract possesses a complex system of defense consisting of immune cells and other nonspecific defense mechanisms. In fact, the GI tract represents the largest immune organ of the body. This chapter provides an overview of the functional anatomy and general principles of regulation in the GI system.
|
| The structure of the GI tract varies greatly from region to region, but there are common features in the overall organization of the tissue. Essentially, the GI tract is a hollow tube divided into major functional segments; the major structures along the tube are the mouth and pharynx, esophagus, stomach, duodenum, jejunum, ileum, colon, rectum, and anus (Fig. 26-1). Together, the duodenum, jejunum, and ileum make up the small intestine, and the colon is sometimes referred to as the large intestine. Associated with the tube are blind-ending glandular structures that are invaginations of the lining of the tube; these glands empty their secretions into the gut lumen (e.g., Brunner's glands in the duodenum, which secrete copious amounts of HCO3-). Additionally, there are glandular organs attached to the tube via ducts through which secretions empty into the gut lumen, for example, the salivary glands and the pancreas.
|
| The major structures along the GI tract have many functions. One important function is storage; the stomach and colon are important storage organs for processed food (sometimes referred to as chyme) and exhibit specialization in terms of both their functional anatomy (e.g., their shape and size) and control mechanisms (characteristics of smooth muscle to produce tonic contractions) that enable them to perform this function efficiently. The predominant function of the small intestine is digestion and absorption; the major specialization of this region of the GI tract is a large surface area over which absorption can occur. The colon reabsorbs water and ions to ensure that they do not get eliminated from the body. Ingested food is moved along the GI tract by the action of muscle in its walls; separating the regions of the GI tract are also specialized muscle structures called sphincters. These function to isolate one region from the next and provide selective retention of contents or prevent backflow, or both.
|
| page 487 |  | | page 488 |
| The blood supply to the intestine is important for carrying absorbed nutrients to the rest of the body. Unlike other organ systems of the body, venous drainage from the GI tract does not return directly to the
heart but first enters the portal circulation leading to the liver. Thus, the liver is unusual in receiving a considerable part of its blood supply from other than the arterial circulation. GI blood flow is also notable for its dynamic regulation; splanchnic blood flow receives about 25% of cardiac output, an amount disproportionate to the mass of the GI tract that it supplies. After a meal, blood can also be diverted from muscle to the GI tract to subserve the metabolic needs of the gut wall and also to remove absorbed nutrients.
|
| Figure 26-1 General anatomy of the GI system and its division into functional segments. |
| The lymphatic drainage of the GI tract is important for the transport of lipid-soluble substances that are absorbed across the GI tract wall. As we will see later, lipids and other lipid-soluble molecules (including some vitamins and drugs) are packaged into particles that are too large to pass into the capillaries and instead pass into lymph vessels in the intestinal wall. These lymph vessels drain into larger lymph ducts, which finally drain into the thoracic duct and thus into the systemic circulation on the arterial side. This has major physiological implications in lipid metabolism and also in the ability of drugs to be delivered straight into the systemic circulation.
|
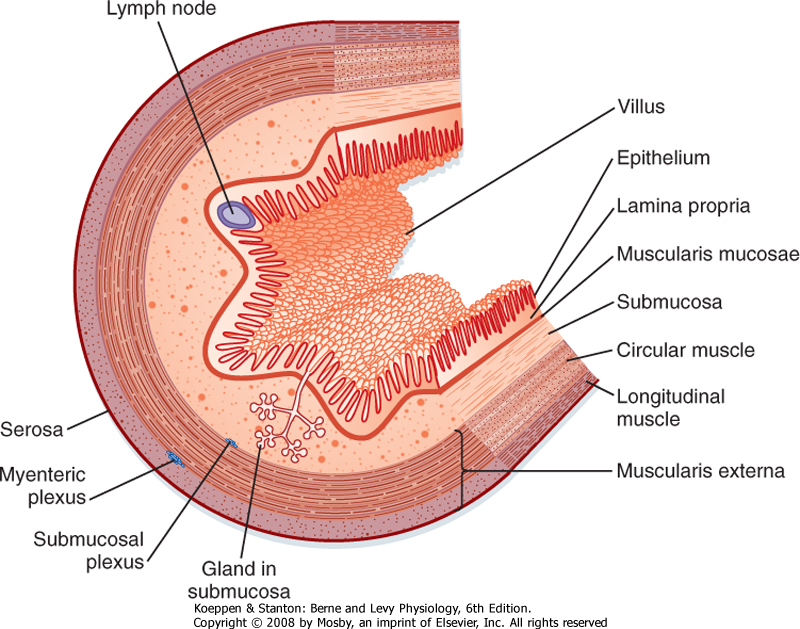
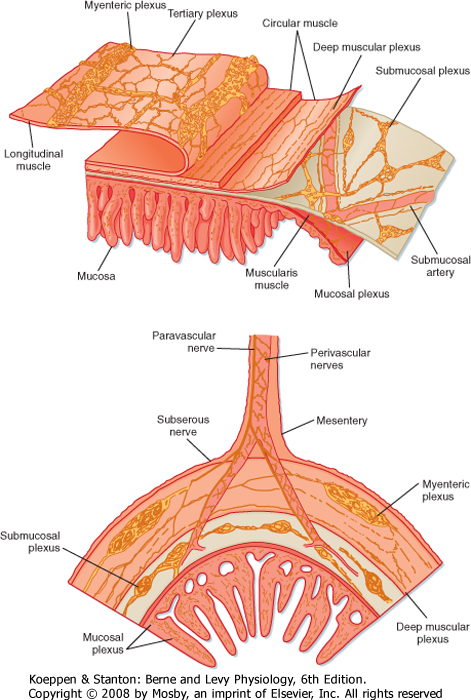
| The wall of the tubular gut is made up of layers consisting of specialized cells (Fig. 26-2).
|
| The mucosa is the innermost layer of the GI tract. It consists of the epithelium, the lamina propria, and the muscularis mucosae. The epithelium is a single layer of specialized cells that line the lumen of the GI tract. It forms a continuous layer along the tube and with the glands and organs that drain into the lumen of the tube. Within this cell layer are a number of specialized epithelial cells; the most abundant are cells termed absorptive enterocytes, which express many proteins important for the digestion and absorption of macronutrients. Enteroendocrine cells contain secretory granules that release regulatory peptides and amines to help regulate GI function. In addition, cells in the gastric mucosa are specialized for the production of protons, and mucin-producing cells throughout the GI tract produce a glycoprotein, mucin, that helps protect the GI tract and lubricate the luminal contents.
|
| Figure 26-2 General organization of the layers composing the wall of the GI tract. |
| page 488 |  | | page 489 |
| The columnar epithelial cells are linked together by intercellular connections called tight junctions. These junctions are complexes of intracellular and transmembrane proteins, and the tightness of these junctions is regulated throughout the postprandial period. The nature of the epithelium varies greatly from one part of the digestive tract to another, depending on the predominant function of that region. For example, the intestinal epithelium is designed for absorption; these cells mediate selective uptake of nutrients, ions, and water. In contrast, the esophagus has a squamous epithelium that has no absorptive role. It is a conduit for the transportation of swallowed food and thus needs some protection from rough food such as fiber, which is provided by the squamous epithelium.
|
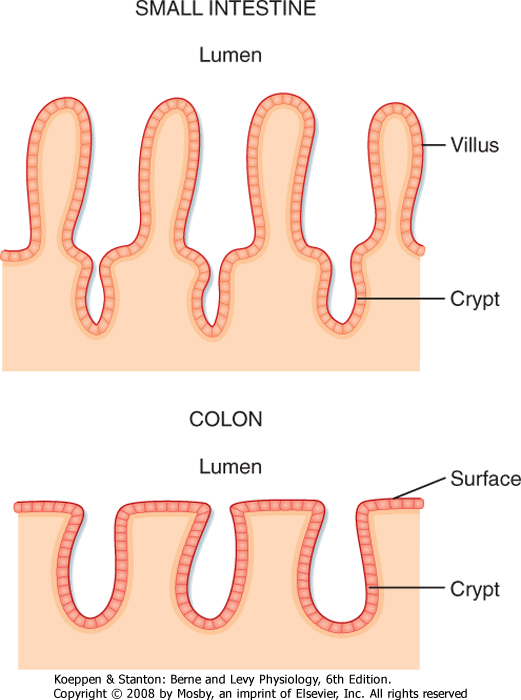
| The surface area of the epithelium is arranged into villi and crypts (Fig. 26-3). Villi are finger-like projections that serve to increase the surface area of the mucosa. Crypts are invaginations or folds in the epithelium. The epithelium lining the GI tract is continuously renewed and replaced by dividing cells; in humans, this process takes about 3 days. These proliferating cells are localized to the crypts, where there is a proliferative zone of intestinal stem cells.
|
| The lamina propria immediately below the epithelium consists largely of loose connective tissue that contains collagen and elastin fibrils. The lamina propria is rich in several types of glands and contains lymph vessels and nodules, capillaries, and nerve fibers. The muscularis mucosae is the thin, innermost layer of intestinal smooth muscle. When seen through an endoscope, the mucosa has folds and ridges that are caused by contractions of the muscularis mucosae.
|
| Figure 26-3 Comparison of the morphology of the epithelium of the small intestine and colon. |
| The next layer is the submucosa. The submucosa consists largely of loose connective tissue with collagen and elastin fibrils. In some regions of the GI tract, glands (invaginations or folds of the mucosa) are present in the submucosa. The larger nerve trunks, blood vessels, and lymph vessels of the intestinal wall lie in the submucosa, together with one of the plexuses of the enteric nervous system (ENS), the submucosal plexus.
|
| The muscularis externa or muscularis propria typically consists of two substantial layers of smooth muscle cells: an inner circular layer and an outer longitudinal layer. Muscle fibers in the circular muscle layer are oriented circumferentially, whereas muscle fibers in the longitudinal muscle layer are oriented along the longitudinal axis of the tube. In humans and most mammals, the circular muscle layer of the small intestine is subdivided into an inner dense circular layer, which consists of smaller, more closely packed cells, and an outer circular layer. Between the circular and longitudinal layers of muscle lies the other plexus of the ENS, the myenteric plexus. Contractions of the muscularis externa mix and circulate the contents of the lumen and propel them along the GI tract.
|
| The wall of the GI tract contains many interconnected neurons. The submucosa contains a dense network of nerve cells called the submucosal plexus (sometimes referred to as Meissner's plexus). The prominent myenteric plexus (Auerbach's plexus) is located between the circular and longitudinal smooth muscle layers. These intramural plexuses constitute the ENS. The ENS helps integrate the motor and secretory activities of the GI system. If the sympathetic and parasympathetic nerves to the gut are cut, many motor and secretory activities continue because these processes are directly controlled by the ENS.
|
| The serosa, or adventitia, is the outermost layer of the GI tract and consists of a layer of squamous mesothelial cells. It is part of the mesentery that lines the surface of the abdominal wall and suspends the organs within the abdominal cavity. The mesenteric membranes secrete a thin, viscous fluid that helps lubricate the abdominal organs so that movement of the organs can occur as the muscle layers contract and relax.
|
| REGULATORY MECHANISMS IN THE GASTROINTESTINAL TRACT
|
| page 489 |  | | page 490 |
| Before we examine the physiology of the GI tract in detail, we will look at the control mechanisms by which function is regulated. Unlike the cardiovascular or respiratory systems, the GI tract undergoes periods of relative quiescence (intermeal period) and periods of intense activity after the intake of food (postprandial period). Consequently, the GI tract has to detect and respond appropriately to the intake of food. In
addition, the macronutrient content of a meal can vary considerably, and there have to be mechanisms that can detect this and mount appropriate physiological responses. Thus, the GI tract has to communicate with associated organs such as the pancreas. Finally, because the GI tract is essentially a long tube, there have to be mechanisms by which events occurring in the proximal portion of the GI tract are signaled to the more distal parts, and vice versa.
|
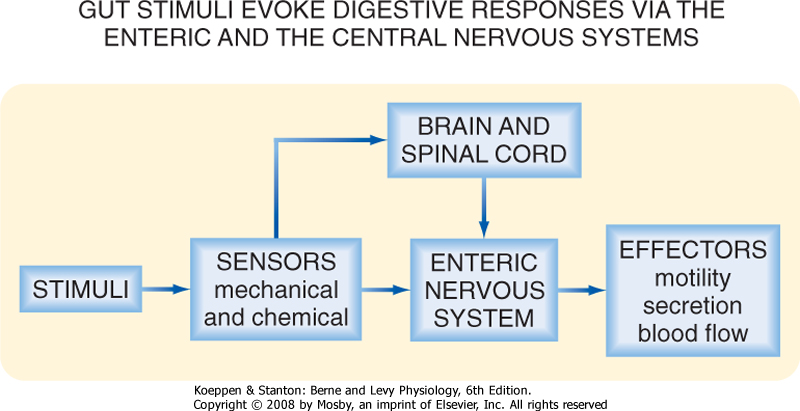
| There are three principal control mechanisms involved in the regulation of GI function: endocrine, paracrine, and neural (Fig. 26-4).
|
| Figure 26-4 The three mechanisms by which function in the GI tract is regulated in the integrated response to a meal. |
| Endocrine regulation describes the process whereby the sensing cell in the GI tract, an enteroendocrine cell (EEC), responds to a stimulus by secreting a regulatory peptide or hormone that travels via the bloodstream to target cells removed from the point of secretion. Cells responding to a GI hormone express specific receptors for the hormone. Hormones released from the GI tract have effects on cells located in other regions of the GI tract and also on glandular structures associated with the GI tract, such as the pancreas. In addition, GI hormones have effects on other tissues that have no direct role in digestion and absorption, including endocrine cells in liver and brain.
|
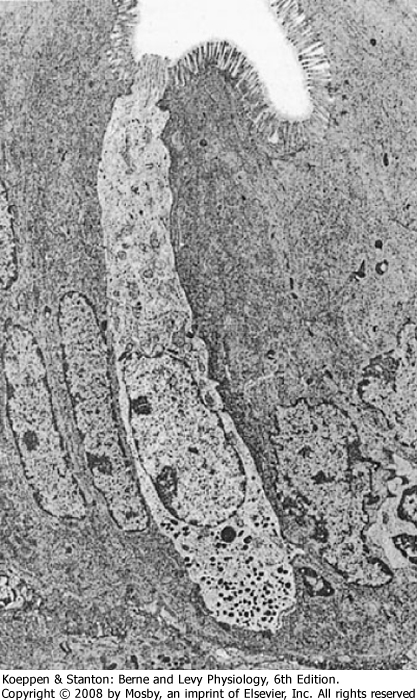
|
| Figure 26-5 Electron micrograph of an open-type endocrine cell in the GI tract. Note the microvilli at the apical projection and the secretory granules in the basolateral portion of the cell. (From Barrett K: Gastrointestinal Physiology [Lange Physiology Series]. New York, McGraw-Hill, 2005.) (Courtesy of Leonard R. Johnson, Ph.D.) |
| page 490 |  | | page 491 |
| EECs are packed with secretory granules, the products of which are secreted from the cell in response to chemical and mechanical stimuli to the wall of the GI tract (Fig. 26-5). In addition, EECs can be stimulated by neural input or other factors not associated with a meal. The most common EECs in the gut wall are referred to as the "open" type; these cells have an apical membrane that is in contact with the lumen of the GI tract (generally regarded as the location where
sensing occurs) and a basolateral membrane through which secretion occurs. There are also "closed"-type EECs that do not have part of their membrane in contact with the luminal surface of the gut; an example is the enterochromaffin-like (ECL) cell in the gastric epithelium, which secretes histamine.
|
| There are many examples of hormones secreted by the GI tract (Table 26-1); it is worth remembering that the first hormone ever identified was the GI hormone secretin. One of the most well characterized GI hormones is gastrin, which is released from endocrine cells located in the wall of the distal part of the stomach. Release of gastrin is stimulated by activation of parasympathetic outflow to the GI tract, and gastrin potently stimulates gastric acid secretion in the postprandial period.
|
| Paracrine regulation describes the process whereby a chemical messenger or regulatory peptide is released from a sensing cell, often an EEC, in the intestinal wall that acts on a nearby target cell by diffusion through the interstitial space. Paracrine agents exert their actions on several different cell types in the wall of the GI tract, including smooth muscle cells, absorptive enterocytes, secretory cells in glands, and even on other EECs. There are several important paracrine agents and they are listed in Table 26-1, along with their site of production, site of action, and function. An important paracrine mediator in the gut wall is histamine. In the stomach, histamine is stored and released by ECL cells located in the gastric glands. Histamine diffuses through the interstitial space in the lamina propria to neighboring parietal cells and stimulates the production of acid. Serotonin (5-hydroxytryptamine [5-HT]), released from enteric neurons, mucosal mast cells, and specialized EECs called enterochromaffin cells, regulates smooth muscle function and water absorption across the intestinal wall. There are other paracrine mediators in the gut wall, including prostaglandins, adenosine, and nitric oxide (NO); the functions of these mediators are not well described, but they are capable of producing changes in GI function.
|
|
Table 26-1.
Hormonal and Paracrine Mediators in the GI Tract |
| GI Hormone | Source | Stimulus for Release | Pathway of Action | Targets | Effect |
| Gastrin | Gastric antrum (G cells) | Oligopeptides | Endocrine | ECL cells and parietal cells of the gastric corpus | Stimulation of parietal cells to secrete H+ and ECL cells to secrete histamine |
| Cholecystokinin | Duodenum (I cells) | Fatty acids, hydrolyzed protein | Paracrine, endocrine | Vagal afferent terminals, pancreatic acinar cells | Inhibition of gastric emptying and H+ secretion; stimulation of pancreatic enzyme secretion, gallbladder contraction, inhibition of food intake |
| Secretin | Duodenum (S cells) | Protons | Paracrine, endocrine | Vagal afferent terminals, pancreatic duct cell | Stimulation of pancreatic ductile secretion (H2O and HCO3-) |
| Gluco-insulinotropic peptide (GIP) | Intestine (K cells) | Fatty acids, glucose | Endocrine | Beta cells of the pancreas | Stimulation of insulin secretion |
| Peptide YY (PYY) | Intestine (L cells) | Fatty acids, glucose, hydrolyzed protein | Endocrine, paracrine | Neurons, smooth muscle | Inhibition of gastric emptying, pancreatic secretion, gastric acid secretion, intestinal motility, food intake |
| Proglucagon-derived peptides 1/2 (GLP-1/2) | Intestine (L cells) | Fatty acids, glucose, hydrolyzed protein | Endocrine, paracrine | Neurons, epithelial cells | Glucose homeostasis, epithelial cell proliferation |
| Many substances can be both paracrine and endocrine regulators of GI function. For example, cholecystokinin, which is released from the duodenum in response to dietary protein and lipid, acts locally on nerve terminals in a paracrine fashion and also affects the pancreas. This will be discussed in more detail in Chapter 29.
|
| Neural Regulation of Gastrointestinal Function
|
| Nerves and neurotransmitters play an important role in regulating the function of the GI tract. In its simplest form, neural regulation occurs when a neurotransmitter is released from a nerve terminal located in the GI tract and the neurotransmitter has an effect on the cell that is innervated. However, in some cases there are no synapses between motor nerves and effector cells in the GI tract. Neural regulation of GI function is very important within an organ, as well as between distant parts of the GI tract.
|
| Posttranslational modification of peptide hormones confers receptor selectivity. |
| There are multiple receptor subtypes for the regulatory peptide hormones released from endocrine cells in the wall of the gut. Their selectivity of action is determined by posttranslational modification of peptide hormones, which then confers receptor selectivity. An example of this is peptide YY (PYY). There are multiple receptor subtypes for PYY, classified as Y1 to Y7. However, not all of them are localized to the gut; Y2 and Y5 are expressed in the GI tract. PYY is released from endocrine cells in the wall of the gut, mainly in response to fatty acids. It is released as a 36-amino acid peptide; however, it can be cleaved to PYY3-36 by the enzyme dipeptidyl peptidase IV, a membrane peptidase. This form of the peptide is selective for the Y2 receptor. Thus, the presence of the enzyme that cleaves the peptide can alter the biological response to PYY secretion. |
| page 491 |  | | page 492 |
| Figure 26-6 Hierarchical neural control of GI function. Stimuli to the GI tract from the meal (e.g., chemical, mechanical, osmotic) will activate both the intrinsic and extrinsic sensory (afferent) pathways, which in turn will activate the extrinsic and intrinsic neural reflex pathways. |
| Glucagon-like peptide 1 (GLP-1) is a regulatory peptide released from EC cells in the gut wall in response to the presence of luminal carbohydrate and lipids. GLP-1 arises from differential processing of the glucagon gene, the same gene that is expressed in the pancreas and that gives rise to glucagon. GLP-1 is involved in regulation of the blood glucose level via stimulation of insulin secretion and also insulin biosynthesis. Agonists of the GLP-1 receptor improve insulin sensitivity in diabetic animal models and human subjects. Administration of GLP-1 also reduces appetite and food intake and delays gastric emptying, responses that may contribute to improving glucose tolerance. Long-acting agonists for the GLP-1 receptor, such as exanatide, have been approved for the treatment of type 2 diabetes. |
 |
| Neural regulation of the GI tract is surprisingly complex. The gut is innervated by two sets of nerves, the extrinsic and intrinsic nervous systems. The extrinsic nervous system is defined as nerves that innervate the gut, with cell bodies located outside the gut wall; these extrinsic nerves are part of the autonomic nervous system (ANS). The intrinsic nervous system, also referred to as the enteric nervous system, has cell bodies that are contained within the wall of the gut (submucosal and myenteric plexuses). Some GI functions are highly dependent on the extrinsic nervous system, yet others can take place independently of the extrinsic nervous system and are mediated entirely by the ENS. However, extrinsic nerves can often modulate intrinsic nervous system function (Fig. 26-6).
|
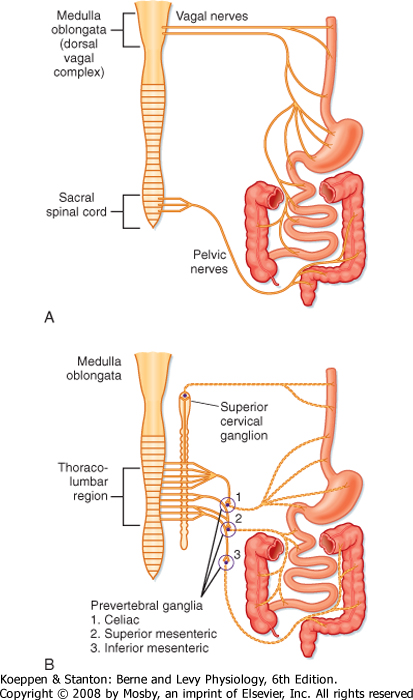
| Figure 26-7 The extrinsic innervation of the GI tract consisting of the parasympathetic (A) and sympathetic (B) subdivisions of the autonomic nervous system. |
| Extrinsic neural innervation to the gut is via the two major subdivisions of the ANS, namely, parasympathetic and sympathetic innervation (Fig. 26-7). Parasympathetic innervation to the gut is via the vagus and pelvic nerves. The vagus nerve, the 10th cranial nerve, innervates the esophagus, stomach, gallbladder, pancreas, first part of the intestine, cecum, and the proximal part of the colon. The pelvic nerves innervate the distal part of the colon and the anorectal
region, in addition to the other pelvic organs that are not part of the GI tract.
|
| page 492 |  | | page 493 |
| Consistent with the typical organization of the parasympathetic nervous system, the preganglionic nerve cell bodies lie in the brainstem (vagus) or the sacral
spinal cord (pelvic). Axons from these neurons run in the nerves to the gut (vagus and pelvic nerves, respectively), where they synapse with postganglionic neurons in the wall of the organ, which in this case are enteric neurons in the gut wall. There is no direct innervation of these efferent nerves to effector cells within the wall of the gut; the transmission pathway is always via a neuron in the ENS.
|
| Consistent with transmission in the ANS, the synapse between preganglionic and postganglionic neurons is an obligatory nicotinic synapse. That is, the synapse between preganglionic and postganglionic neurons is mediated via acetylcholine released from the nerve terminal and acting at nicotinic receptors localized on the postganglionic neuron, which in this case is an intrinsic neuron.
|
| Sympathetic innervation is supplied by cell bodies in the spinal cord and fibers that terminate in the prevertebral ganglia (celiac, superior, and inferior mesenteric ganglia); these are the preganglionic neurons. These nerve fibers synapse with postganglionic neurons in the ganglia, and the fibers leave the ganglia and reach the end organ along the major blood vessels and their branches. Rarely, there is a synapse in the paravertebral (chain) ganglia, as seen with sympathetic innervation of other organ systems. Some vasoconstrictor sympathetic fibers directly innervate blood vessels of the GI tract, and other sympathetic fibers innervate glandular structures in the wall of the gut.
|
| The ANS, both parasympathetic and sympathetic, also carries the fibers of afferent (toward the central nervous system [CNS]) neurons; these are sensory in nature. The cell bodies for the vagal afferents are in the nodose ganglion. These neurons have a central projection terminating in the nucleus of the tractus solitarius in the brainstem and the other terminal in the gut wall. The cell bodies of the spinal afferent neurons that run with the sympathetic pathway are segmentally organized and are found in the dorsal root ganglia. Peripheral terminals of the spinal and vagal afferents are located in all layers of the gut wall, where they detect information about the state of the gut. Afferent neurons send this information to the CNS. Information sent to the CNS relays the nature of the luminal contents, such as acidity, nutrient content, and osmolality of the luminal contents, as well as the degree of stretch or contraction in smooth muscle. Afferent innervation is also responsible for transmitting painful stimuli to the CNS.
|
| The components of a reflex pathway-afferents, interneurons, and efferent neurons-exist within the extrinsic innervation to the GI tract. These reflexes can be mediated entirely via the vagus nerve (termed a vagovagal reflex), which has both afferent and efferent fibers. The vagal afferents send sensory information to the CNS, where they synapse with an interneuron, which then drives activity in the efferent motor neuron. These extrinsic reflexes are very important in the regulation of GI function after the ingestion of a meal. An example of an important vagovagal reflex is the gastric receptive relaxation reflex, in which distention of the stomach results in relaxation of the smooth muscle in the stomach; this allows filling of the stomach to occur without an increase in intraluminal pressure.
|
| In general, as with other visceral organ systems, the parasympathetic and sympathetic nervous systems tend to work in opposition. However, this is not as simple as in the cardiovascular system, for example. Activation of the parasympathetic nervous system is important in the integrative response to a meal, and we will look at many examples of this in the following chapters. The parasympathetic nervous system generally results in the activation of physiological processes in the gut wall, although there are notable exceptions. In contrast, the sympathetic nervous system tends to be inhibitory to GI function and is more frequently activated in pathophysiological circumstances. Overall, sympathetic activation inhibits smooth muscle function; the exception to this is the sympathetic innervation of GI sphincters, in which sympathetic activation tends to induce contraction of smooth muscle. Moreover, the sympathetic nervous system is notably important in regulation of blood flow in the GI tract.
|
| Intrinsic Neural Innervation
|
| The ENS is made up of two major plexuses, which are collections of nerve cell bodies (ganglia) and their fibers, all originating in the wall of the gut (Fig. 26-8). The myenteric plexus lies between the longitudinal and circular muscle layers and the submucosal plexus lies in the submucosa. Neurons in the two plexuses are linked by interganglionic strands.
|
| Neurons in the ENS are characterized functionally as afferent neurons, interneurons, or efferent neurons, similar to neurons in the extrinsic part of the ANS. Thus, all components of a reflex pathway can be contained within the ENS. Stimuli in the wall of the gut are detected by afferent neurons, which activate interneurons and then efferent neurons to alter function. In this way the ENS can act autonomously from extrinsic innervation. However, neurons in the ENS, as we have already seen, are innervated by extrinsic neurons, and thus the function of these reflex pathways can be modulated by the extrinsic nervous system. Because the ENS is capable of performing its own integrative functions and complex reflex pathways, it is sometimes referred to as the "little brain in the gut" as a result of its importance and complexity. It is estimated that there are as many neurons in the ENS as in the spinal cord. In addition, many GI hormones also act as neurotransmitters in the ENS and in the brain in regions involved in autonomic outflow. These mediators and regulatory peptides are thus referred to as "brain-gut peptides," and the extrinsic and intrinsic components innervating the gut are sometimes referred to as the "brain-gut axis."
|
| RESPONSE OF THE GI TRACT TO A MEAL
|
| page 493 |  | | page 494 |
| Figure 26-8 The enteric nervous system in the wall of the GI tract. |
| Hirschsprung's disease is a congenital disorder of the enteric nervous system characterized by failure to pass meconium at birth or severe chronic constipation in infancy. The typical features are absence of myenteric and submucosal neurons in the distal part of the colon and rectum. It is a polygenic disorder with characteristic mutations in at least three different classes of genes involved in neuronal development and differentiation. |
 |
| This introductory chapter provides a broad overview of the anatomy and regulatory mechanisms in the GI tract. In the following chapters there will be discussion
of the integrated response to a meal in order to provide the details of GI physiology. The response to a meal is classically divided into phases: cephalic, oral, esophageal, gastric, duodenal, and intestinal. In each phase the meal presents certain stimuli (e.g., chemical, mechanical, and osmotic) that activate different pathways (neural, paracrine, and humoral reflexes) that result in changes in effector function (secretion and motility). There is considerable crosstalk between the regulatory mechanisms that have been outlined, and this will be discussed in the next chapters. As with maintenance of homeostasis in other systems of the body, control of GI function requires complex regulatory mechanisms to sense and act in a dynamic fashion.
|
| page 494 |  | | page 495 |
- The GI tract is a tube subdivided into regions that subserve different functions associated with digestion and absorption.
- The lining of the GI tract is subdivided into layers-the mucosal, submucosal, and muscle layers.
- There are three major control mechanisms: hormonal, paracrine, and neural.
- The innervation of the GI tract is particularly interesting because it consists of two interacting components, extrinsic and intrinsic.
- Extrinsic innervation (cell bodies outside the wall of the GI tract) consists of the two subdivisions of the ANS: parasympathetic and sympathetic. Both have an important sensory (afferent) component.
- The intrinsic or enteric nervous system (cell bodies in the wall of the GI tract) can act independently of extrinsic neural innervation.
- When a meal is in different regions of the tract, sensory mechanisms detect the presence of the nutrients and mount appropriate physiological responses in that region of the tract, as well as in more distal regions. These responses are mediated by endocrine, paracrine, and neural pathways.
|
 |
|