| 28 The Gastric Phase of the Integrated Response to a Meal
|
| In this chapter we will study gastrointestinal (GI) tract physiology when food is in the stomach (i.e., the gastric phase of digestion). This chapter discusses gastric function and its regulation, in addition to changes in function that occur in more distal regions of the GI tract. The main functions of the stomach are to act as a temporary reservoir for the meal and to initiate protein digestion through the secretion of acid and the enzyme precursor pepsinogen. Other functions are listed in Table 28-1.
|
| Food entering the stomach from the esophagus causes mechanical stimulation of the gastric wall via distention and stretching of smooth muscle. Food, predominantly oligopeptides and amino acids, also provides chemical stimulation when present in the gastric lumen. Regulation of gastric function during the gastric phase is dependent on endocrine, paracrine, and neural pathways. These pathways are activated by mechanical and chemical stimuli, which result in both intrinsic and extrinsic neural reflex pathways that are important for the regulation of gastric function. Afferent neurons that pass from the GI tract to the central nervous system (and to a lesser extent to the spinal cord) via the vagus nerve respond to these mechanical and chemical stimuli and activate parasympathetic outflow.
|
| The endocrine pathways include the release of gastrin, which stimulates gastric acid secretion, and the release of somatostatin, which inhibits gastric secretion. Important paracrine pathways include histamine release, which stimulates gastric acid secretion. The responses elicited by activation of these pathways include both secretory and motor responses; secretory responses include secretion of acid, pepsinogen, mucus, intrinsic factor, gastrin, lipase, and HCO3-. Overall, these secretions initiate protein digestion and protect the gastric mucosa. Motor responses (changes in activity of smooth muscle) include inhibition of motility of the proximal part of the stomach (receptive relaxation) and stimulation of motility of the distal part of the stomach, which causes antral peristalsis. These changes in motility play important roles in storage and mixing of the meal with secretions and are also involved in regulating the flow of contents out of the stomach.
|
| FUNCTIONAL ANATOMY OF THE STOMACH
|
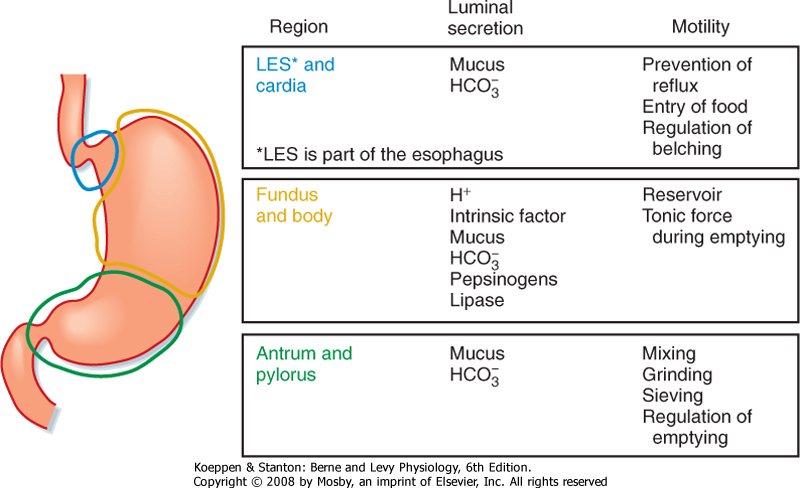
| The stomach is divided into three regions: the cardia, the corpus (also referred to as the fundus or body), and the antrum (Fig. 28-1). However, when discussing the physiology of the stomach, it is helpful to think of it as subdivided into two functional regions: the proximal and distal parts of the stomach. The proximal portion of the stomach (called proximal because it is the most cranial) and the distal portion of the stomach (furthest away from the mouth) have quite different functions in the postprandial response to a meal, which will be discussed later.
|
| The lining of the stomach is covered with a columnar epithelium folded into gastric pits; each pit is the opening of a duct into which one or more gastric glands empty (Fig. 28-2). The gastric pits account for a significant fraction of the total surface area of the gastric mucosa. The gastric mucosa is divided into three distinct regions based on the structure of the glands. The small cardiac glandular region, located just below the lower esophageal sphincter (LES), primarily contains mucus-secreting gland cells. The remainder of the gastric mucosa is divided into the oxyntic or parietal (acid-secreting) gland region, located above the gastric notch (equivalent to the proximal part of the stomach), and the pyloric gland region, located below the notch (equivalent to the distal part of the stomach).
|
| page 504 |  | | page 505 |
| Figure 28-1 The three functional regions of the stomach. The regions have different luminal secretions and patterns of smooth muscle activity indicative of their unique functions in response to food. |
| Figure 28-2 Representation of the structure of the gastric mucosa showing a section through the wall of the stomach (A) and detail of the structure of gastric glands and cell types in the mucosa (B). |
|
Table 28-1.
Functions of the Stomach |
| Storage-acts as temporary reservoir for the meal |
| Secretion of H+ to kill microorganisms and convert pepsinogen to its active form |
| Secretion of intrinsic factor to absorb vitamin B12 (cobalamin) |
Secretion of mucus and
 to protect the gastric mucosa to protect the gastric mucosa |
| Secretion of water for lubrication and to provide aqueous suspension of nutrients |
| Motor activity for mixing secretions (H+ and pepsin) with ingested food |
| Coordinated motor activity to regulate the emptying of contents into the duodenum |
| The structure of a gastric gland from the oxyntic glandular region is illustrated in Figure 28-2. Surface epithelial cells extend slightly into the duct opening. The opening of the gland is called the isthmus and is lined with surface mucous cells and a few parietal cells. Mucous neck cells are located in the narrow neck of the gland. Parietal or oxyntic cells, which secrete HCl and intrinsic factor (involved in absorption of vitamin B12), and chief or peptic cells, which secrete pepsinogens, are located deeper in the gland. Oxyntic glands also contain enterochromaffin-like (ECL) cells, which secrete histamine, and D cells, which secrete somatostatin. Parietal cells are particularly numerous in glands in the fundus, whereas mucus-secreting cells are more numerous in glands of the pyloric (antral)
glandular region. In addition, the pyloric glands contain G cells, which secrete the hormone gastrin. The parietal glands can also be divided into regions: the neck (neck mucous cells and parietal cells) and the base (peptic/chief and parietal cells). Endocrine cells are scattered throughout the glands.
|
| page 505 |  | | page 506 |
| The fluid secreted into the stomach is called gastric juice. Gastric juice is a mixture of the secretions of the surface epithelial cells and the secretions of gastric
glands. One of the most important components of gastric juice is H+, a secretion that occurs in the face of a very large concentration gradient. Thus, H+ secretion by the parietal mucosa is an energy-intensive process. The cytoplasm of the parietal cell is densely packed with mitochondria, which have been estimated to fill 30% to 40% of the cell's volume. One major function of H+ is conversion of inactive pepsinogen (the major enzyme product of the stomach) to pepsins, which initiate protein digestion in the stomach. Additionally, ions are important for preventing invasion and colonization of the gut by bacteria and other pathogens that may be ingested with food. The stomach also secretes significant amounts of HCO3- and mucus, important for protection of the gastric mucosa against the acidic and peptic luminal environment. However, in a healthy human the only gastric secretion required is intrinsic factor, which is necessary for the absorption of vitamin B12 (cobalamin). The functions of other components of gastric juice are redundant with secretions provided more distally in the GI tract.
|
| Composition of Gastric Secretions
|
| Like other GI secretions, gastric juice consists of inorganic and organic constituents together with water. Among the important components of gastric juice are HCl, salts, pepsins, intrinsic factor, mucus, and HCO3-. Secretion of all these components increases after a meal.
|
| Inorganic Constituents of Gastric Juice
|
| The ionic composition of gastric juice depends on the rate of secretion. The higher the secretory rate, the higher the concentration of H+ ions. At lower secretory rates, [H+] decreases and [Na+] increases. [K+] is always higher in gastric juice than in plasma. Consequently, prolonged vomiting may lead to hypokalemia. At all rates of secretion, Cl- is the major anion of gastric juice. At high rates of secretion, gastric juice resembles an isotonic solution of HCl. Gastric HCl converts pepsinogens to active pepsins and provides the acid pH at which pepsins are active.
|
| The rate of gastric H+ secretion varies considerably among individuals. In humans, basal (unstimulated) rates of gastric H+ production typically range from about 1 to 5 mEq/hr. During maximal stimulation, HCl production rises to 6 to 40 mEq/hr. The basal rate is greater at night and lowest in the early morning. The total number of parietal cells in the stomach of normal individuals varies greatly, and this variation is partly responsible for the wide range in basal and stimulated rates of HCl secretion.
|
| Organic Constituents of Gastric Juice
|
|
Table 28-2.
Stimulation of Chief Cells in the Integrated Response to a Meal |
| Stimulant | Source |
| Acetylcholine (ACh) | Enteric neurons |
| Gastrin | G cells in the gastric antrum |
| Histamine | ECL cells in the gastric corpus |
| Cholecystokinin (CCK) | I cells in the duodenum |
| Secretin | S cells in the duodenum |
| The predominant organic constituent of gastric juice is pepsinogen, the inactive proenzyme of pepsin. Pepsins, often collectively called "pepsin," are a group of proteases secreted by the chief cells of the gastric glands. Pepsinogens are contained in membrane-bound zymogen granules in the chief cells. Zymogen granules release their contents by exocytosis when chief cells are stimulated to secrete (Table 28-2). Pepsinogens
are converted to active pepsins by the cleavage of acid-labile linkages. The lower the pH, the more rapid the conversion. Pepsins also act proteolytically on pepsinogens to form more pepsin. Pepsins are most proteolytically active at pH 3 and below. Pepsins may digest as much as 20% of the protein in a typical meal but are not required for digestion because their function can be replaced by that of pancreatic proteases. When the pH of the duodenal lumen is neutralized, pepsins are inactivated by the neutral pH.
|
| Intrinsic factor, a glycoprotein secreted by parietal cells of the stomach, is required for the normal absorption of vitamin B12. Intrinsic factor is released in response to the same stimuli that elicit the secretion of HCl by parietal cells. Secretion of intrinsic factor is the only gastric function that is essential for human life.
|
| Cellular Mechanisms of Gastric Acid Secretion
|
| Parietal cells have a distinctive ultrastructure (Fig. 28-3). Branching secretory canaliculi course through the cytoplasm and are connected by a common outlet to the cell's luminal surface. Microvilli line the surfaces of the secretory canaliculi. The cytoplasm of unstimulated parietal cells contains numerous tubules and vesicles, which is called the tubulovesicular system. The membranes of tubulovesicles contain the transport proteins responsible for secretion of H+ and Cl- into the lumen of the gland. When parietal cells are stimulated to secrete HCl (Fig. 28-3), tubulovesicular membranes fuse with the plasma membrane of the secretory canaliculi. This extensive membrane fusion greatly increases the number of H+-K+ antiporters in the plasma membrane of the secretory canaliculi. When parietal cells secrete gastric acid at the maximal rate, H+ is pumped against a concentration gradient that is about 1 million-fold. Thus, the pH is 7 in the parietal cell cytosol and 1 in the lumen of the gastric gland.
|
| page 506 |  | | page 507 |
| Figure 28-3 Parietal cell ultrastructure. A, A resting parietal cell showing the tubulovesicular apparatus in the cytoplasm and the intracellular canaliculus. B, An activated parietal cell that is secreting acid. The tubulovesicles have fused with the membranes of the intracellular canaliculus, which is now open to the lumen of the gland and lined with abundant long microvilli. |
| Figure 28-4 Mechanism of H+ and Cl- secretion by an activated parietal cell in the gastric mucosa. |
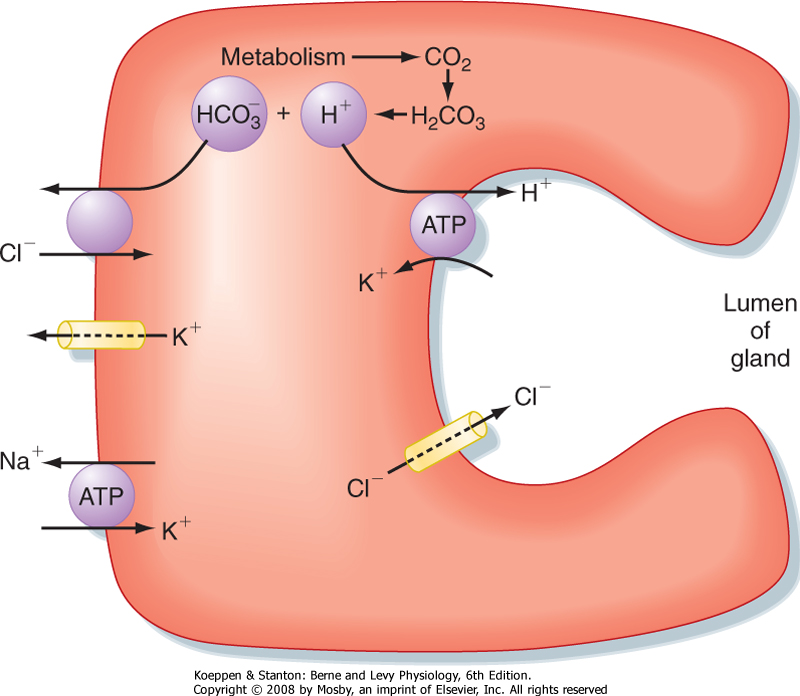
| The cellular mechanism of H+ secretion by the parietal cell is depicted in Figure 28-4. Cl- enters the cell across the basolateral membrane in exchange for HCO3- generated in the cell by the action of carbonic anhydrase, which produces HCO3- and H+. H+ is secreted across the luminal membrane by H+,K+-ATPase in exchange for K+. Cl- enters the lumen via an ion channel (a ClC Cl- channel) located in the luminal membrane. Increased intracellular Ca++ and cAMP stimulate luminal membrane conduction of Cl- and K+.
Increased K+ conductance hyperpolarizes the luminal membrane potential, which increases the driving force for efflux of Cl- across the luminal membrane. The K+ channel in the basolateral membrane also mediates the efflux of K+ that accumulates in the parietal cell via the activity of H+,K+-ATPase. In addition, cAMP and Ca++ promote the trafficking of Cl- channels into the luminal membrane and the fusion of cytosolic tubulovesicles containing H+,K+-ATPase with the membrane of the secretory canaliculi (Figs. 28-3 and 28-4). Parietal cell secretion of H+is also accompanied by transport of HCO3- into the bloodstream to maintain intracellular pH.
|
| The surface epithelial cells also secrete a watery fluid that contains Na+ and Cl- in concentrations similar to those in plasma, but with higher K+ and HCO3- concentrations. HCO3- is entrapped by the viscous mucus that coats the surface of the stomach; thus, the mucus secreted by the resting mucosa lines the stomach with a sticky, alkaline coat. When food is eaten, moreover, rates of secretion of both mucus and HCO3- increase.
|
| Secretions that contain mucins are viscous and sticky and are collectively termed mucus. Mucins are secreted by mucous neck cells located in the necks of gastric glands and by the surface epithelial cells of the stomach. Mucus is stored in large granules in the apical cytoplasm of mucous neck cells and surface epithelial cells and is released by exocytosis.
|
| page 507 |  | | page 508 |
| Figure 28-5 Schematic representation of the structure of gastric mucins before and after hydrolysis by pepsin. Intact mucins are tetramers of four similar monomers of about 500,000 Da. Each monomer is largely covered by carbohydrate side chains that protect it from proteolytic degradation. The central portion of the mucin tetramer, near the disulfide cross-links, is more susceptible to proteolytic digestion. Pepsins cleave bonds near the center of the tetramers to release fragments about the size of monomers. |
| Gastric mucins are about 80% carbohydrate by weight and consist of four similar monomers of about
500,000 Da each that are linked together by disulfide bonds (Fig. 28-5). These tetrameric mucins form a sticky gel that adheres to the surface of the stomach. However, this gel is subject to proteolysis by pepsins, which cleave disulfide bonds near the center of the tetramers. Proteolysis releases fragments that do not form gels and thus dissolves the protective mucus layer. Maintenance of the protective mucus layer requires continuous synthesis of new tetrameric mucins to replace the mucins that are cleaved by pepsins.
|
| Mucus is secreted at a significant rate in the resting stomach. Secretion of mucus is stimulated by some of the same stimuli that enhance acid and pepsinogen secretion, especially acetylcholine released from parasympathetic nerve endings near the gastric glands. If the gastric mucosa is mechanically deformed, neural reflexes are evoked to enhance mucus secretion.
|
| Regulation of Gastric Secretion
|
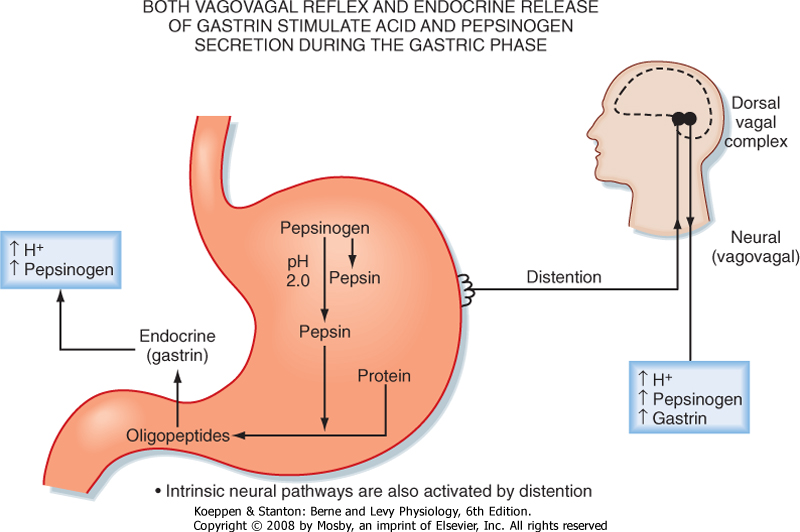
| Parasympathetic innervation via the vagus nerve is the strongest stimulant of gastric H+ secretion. Extrinsic efferent fibers terminate on intrinsic neurons that innervate parietal cells, ECL cells that secrete the paracrine mediator histamine, and endocrine cells that secrete the hormone gastrin. In addition, vagal stimulation results in the secretion of pepsinogen, mucus, HCO3-, and intrinsic factor. Stimulation of the parasympathetic nervous system also occurs during the cephalic and oral phase of the meal. However, the gastric phase produces the largest stimulation of gastric secretion of the postprandial period (Fig. 28-6).
|
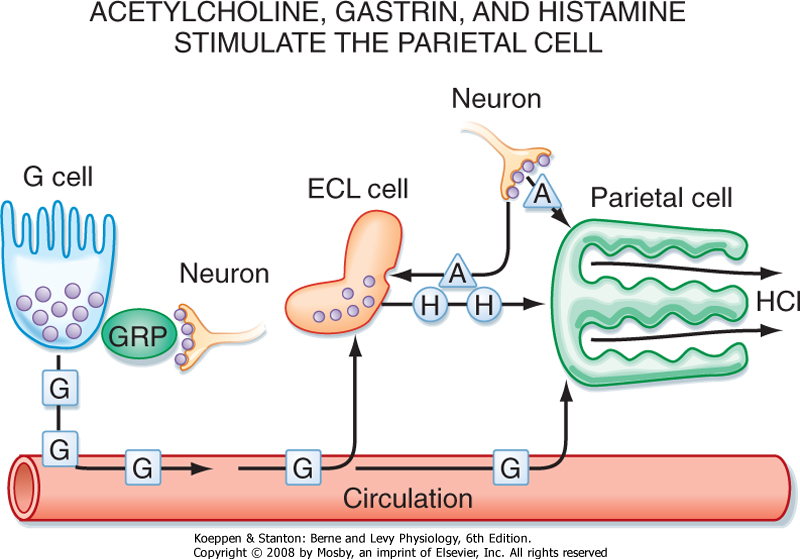
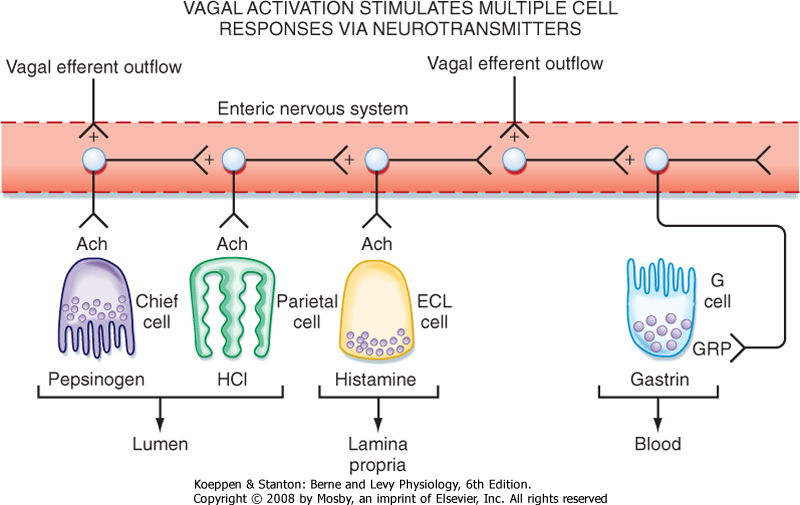
| Stimulation of gastric acid secretion is an excellent example of a "feed forward" (or cascade) response that uses endocrine, paracrine, and neural pathways. Activation of intrinsic neurons by vagal efferent activity results in the release of acetylcholine from nerve terminals, which activates cells in the gastric epithelium. Parietal cells express muscarinic receptors and are activated to secrete H+ in response to vagal efferent nerve activity. In addition, parasympathetic activation, via gastrin-releasing peptide from intrinsic neurons, releases gastrin from G cells located in the gastric glands in the gastric antrum (Fig. 28-6). Gastrin enters the bloodstream and, via an endocrine mechanism, further stimulates the parietal cell to secrete H+. Parietal cells express cholecystokinin type 2 (CCK2) receptors for gastrin. Histamine is also secreted in response to vagal nerve stimulation, and ECL cells express muscarinic and gastrin receptors. Thus, gastrin and vagal efferent activity induce the release of histamine, which potentiates the effects of both gastrin and acetylcholine on the parietal cell. Hence, activation of parasympathetic (vagal) outflow to the stomach is very efficient at stimulating the parietal cell to secrete acid (Fig. 28-7).
|
| In the gastric phase, the presence of food in the stomach is detected and activates vagovagal reflexes to stimulate secretion. Food in the stomach results in distention and stretch, which are detected by afferent (or sensory) nerve endings in the gastric wall. These are the peripheral terminals of vagal afferent nerves that transmit information to the brainstem and thereby drive activity in vagal efferent fibers, a vagovagal reflex (Fig. 28-6). In addition, digestion of proteins increases the concentration of oligopeptides and free amino acids in the lumen, which are detected by chemosensors in the gastric mucosa. Oligopeptides and amino acids also stimulate vagal afferent activity. The exact nature of the chemosensors is not clear but may involve endocrine cells that release their contents to activate nerve endings. This topic will be discussed in more detail in Chapter 29.
|
| page 508 |  | | page 509 |
| Figure 28-6 Neural regulation of gastric acid secretion in the gastric phase of the meal is mediated by the vagus nerve. The stimulation that occurs in the cephalic and oral phases, before food reaches the stomach, results in stimulation of parietal cells to secrete acid and chief cells to secrete pepsinogen. Thus, when food reaches the stomach, protein digestion is initiated by generating protein hydrolysate, which further stimulates the secretion of gastrin from the mucosa of the gastric antrum. In addition, gastric distention activates a vagovagal reflex that further stimulates gastric acid and pepsinogen secretion. |
|
| Figure 28-7 The parietal cell is regulated by neural, hormonal, and paracrine pathways. Activation of vagal parasympathetic preganglionic outflow to the stomach acts in three ways to stimulate gastric acid secretion. There is direct neural innervation and activation of the parietal cell via release of acetylcholine (ACh) from enteric neurons, which acts on the parietal cell via muscarinic receptors. In addition, neural activation of the ECL cell stimulates the release of histamine, which acts via a paracrine pathway to stimulate the parietal cell. Finally, G cells located in gastric glands in the gastric antrum are activated by the release of gastrin-releasing peptide from enteric neurons, which acts on the G cell to stimulate the release of gastrin. Gastrin thereafter acts via a humoral pathway to stimulate the parietal cell. |
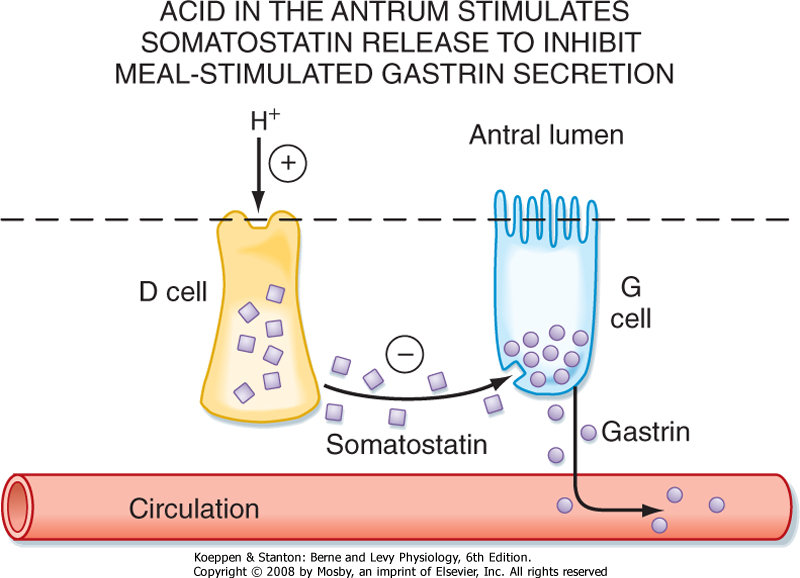
| There is also an important negative-feedback mechanism whereby the presence of acid in the distal part of the stomach (antrum) induces a feedback loop to inhibit the parietal cell such that meal-stimulated H+ secretion does not go unchecked. When the concentration of H+ in the lumen reaches a certain threshold (below pH 3), somatostatin is released from endocrine
cells in the antral mucosa. Somatostatin has a paracrine action on neighboring G cells to decrease the release of gastrin and thereby decrease gastric acid secretion (Fig. 28-8).
|
| Figure 28-8 Feedback regulation of gastric acid secretion by release of somatostatin and its action on G cells in the gastric antrum. Endocrine cells in the mucosa of the gastric antrum sense the presence of H+ and secrete somatostatin. This in turn acts on specific receptors on G cells to inhibit the release of gastrin and thus bring about inhibition of gastric acid secretion. |
| page 509 |  | | page 510 |
| Figure 28-9 Vagal parasympathetic stimulation of gastric secretions via enteric neurons. Vagal preganglionic neurons innervate the myenteric and submucosal plexus; the terminals of the vagal preganglionic neurons innervate many enteric neurons and thus bring about changes in function as described in Figure 28-7. |

|
| Figure 28-10 Signal transduction mechanisms showing the mechanism of action of agonists (secretagogues) and antagonists that regulate secretion in parietal cells. Acetylcholine (ACh) binds to muscarinic M3 receptors. Histamine acts via the H2 receptor. Gastrin binds to the cholecystokinin type 2 (CCK2) receptor. Activation of M2 and CCK2 receptors results in opening of Ca++ channels and release of Ca++ from intracellular stores and thus an increase in cytosolic [Ca++]. Activation of H2 receptors activates adenylyl cyclase to increase intracellular levels of cAMP. Ac, adenylyl cyclase; ACh, acetylcholine; CCK, cholecystokinin; DAG, diacylglycerol; EGF, epidermal growth factor; IP3, inositol triphosphate; PGE2, prostaglandin E2; PIP2, phosphatidylinositol 4,5-diphosphate; PKC, protein kinase C; PLC, protein lipase C; TGF-α, transforming growth factor α. |
| The receptors on the parietal cell membrane for acetylcholine, gastrin, and histamine, as well as the intracellular second messengers by which these secretagogues
act, are shown in Figure 28-9. Histamine is the strongest agonist of H+ secretion, whereas gastrin and acetylcholine are much weaker agonists. However, histamine, acetylcholine, and gastrin potentiate one another's actions on the parietal cell. Antagonists of H2 histamine receptors, such as cimetidine, block secretagogue-stimulated acid secretion. Thus, much of the response to gastrin results from gastrin-stimulated release of histamine. Gastrin also has important trophic effects: elevation of gastrin levels causes ECL cells to increase in size and number. Binding of histamine to H2 receptors on parietal cell plasma membranes activates adenylyl cyclase and elevates the
cytosolic concentration of cAMP. These events stimulate H+ secretion by activating basolateral K+ channels and apical Cl- channels and by causing more H+,K+-ATPase molecules and Cl- channels to be inserted into the apical plasma membrane (Fig. 28-4). Acetylcholine binds to M3 muscarinic receptors and opens Ca++ channels in the apical plasma membrane. Acetylcholine also elevates intracellular [Ca++] by promoting the release of Ca++ from intracellular stores, which enhances H+ secretion by activating basolateral K+ channels and by causing more H+,K+-ATPase molecules and Cl- channels to be inserted into the apical plasma membrane. Gastrin enhances acid secretion by binding to CCK-B receptors (Fig. 28-10).
|
| page 510 |  | | page 511 |
| Some digestion of nutrients occurs in the stomach. However, this is not required for full digestion of a meal because intestinal digestion is sufficient. Some amylase-mediated digestion of carbohydrates occurs in the stomach. Amylase is sensitive to pH and is inactivated at low pH; however, some amylase is active even in the acidic gastric environment of the stomach because of substrate protection. Thus, when carbohydrate occupies the active site of amylase, it protects the enzyme from degradation.
|
| The digestion of lipids also starts in the stomach. The mixing patterns of gastric motility result in the formation of an emulsion of lipids and gastric lipase, which attaches to the surface of lipid droplets in the emulsion and generates free fatty acids and monoglyceride from dietary triglyceride. However, the extent of hydrolysis of triglyceride is approximately 10%, and such hydrolysis is not essential for normal digestion and absorption of dietary lipids. Moreover, as discussed in the next chapter, the products of lipolysis are not available for absorption in the stomach because of its low luminal pH.
|
| Gastric Mucosal Protection and Defense
|
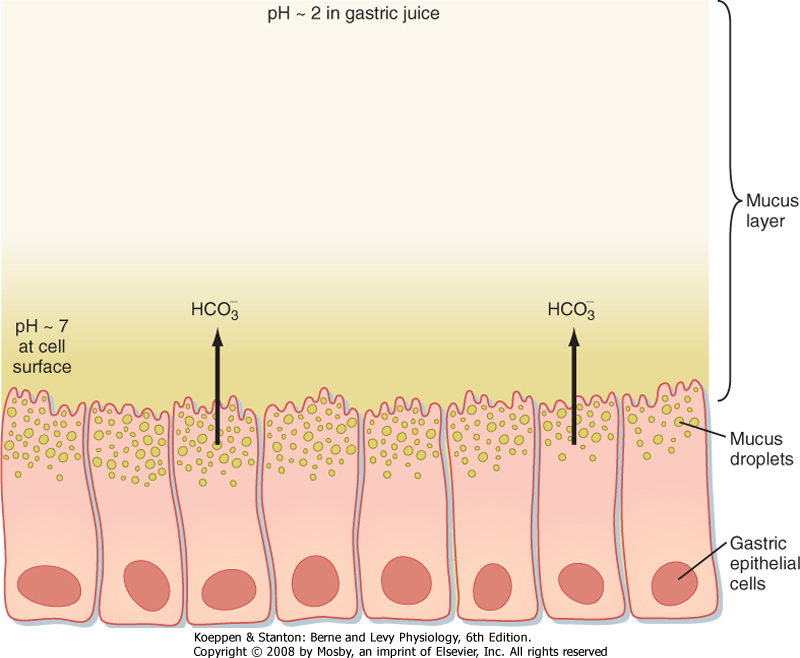
| Figure 28-11 The surface of the stomach is protected by the gastric mucosal barrier. Buffering by the HCO3--rich secretions and the restraint to convective mixing caused by the high viscosity of the mucus layer allow the pH at the cell surface to remain near 7, whereas the pH in the gastric juice in the lumen is 1 to 2. |
| Mucus and HCO3- protect the surface of the stomach from the effects of H+ and pepsins. The protective mucus gel that forms on the luminal surface of the stomach, as well as alkaline secretions entrapped within it, constitute a gastric mucosal barrier that prevents damage to the mucosa by the gastric contents (Fig. 28-11). The mucus gel layer, which is about 0.2 mm thick, effectively separates the HCO3--rich
secretions of the surface epithelial cells from the acidic contents of the gastric lumen. The mucus allows the pH of epithelial cells to be maintained at nearly neutral despite a luminal pH of about 2. Mucus also slows the diffusion of acid and pepsins to the epithelial cell surface. Protection of the gastric epithelium depends on both mucus and HCO3- secretion.
|
| GASTROINTESTINAL MOTILITY
|
| To understand GI motility, it is necessary to review some properties of smooth muscle function. The motion of the gut wall governs the flow of the luminal contents along its length; the main patterns of motility are mixing (segmentation) and propulsion (peristalsis). In addition, smooth muscle activity in the stomach and colon subserves a storage function.
|
| Functional Anatomy of Gastrointestinal Smooth Muscle
|
| page 511 |  | | page 512 |
| There are times when the gastric mucosal barrier fails. Superficial breakdowns of the GI lining not involving the submucosa are called erosions. They generally heal without intervention. In contrast, breakdowns of the GI lining involving the muscularis and deeper layers are called ulcers. Gastric and duodenal erosions and ulcers occur as a result of an imbalance between the mechanisms that protect the mucosa and aggressive factors that can break it down. A healthy stomach/duodenum has ample natural protection against the destructive effects of H+. Factors that magnify the harmful effect of H+ on the stomach/duodenum or act separately from H+ include pepsin, bile, the bacterium Helicobacter pylori, and the class of drugs known as nonsteroidal antiinflammatory drugs (NSAIDs). Indeed, ulcer disease is becoming more common as the population ages and has more need of NSAIDs for non-GI complaints such as arthritis. Alcohol, tobacco, and caffeine are also risk factors for ulcers. Infectious agents can also cause gastritis (inflammation of the gastric epithelium). H. pylori is a spiral bacterium that has now become widely recognized as one factor that can lead to gastritis, ulcer formation, and in humans, gastric carcinoma. H. pylori exists in the stomach because it secretes an enzyme, urease, that converts urea to NH3, which is used to buffer H+ by forming NH4+. An aggressive regimen of antibiotic treatment, sometimes in combination with an H+,K+-ATPase inhibitor, can often eliminate the infection, after which the gastritis and ulcer symptoms improve. |
 |
| The smooth muscle in the GI tract is similar in structure to other smooth muscle found in the body. Fusiform cells are packed together in bundles surrounded by a connective tissue sheath. Gap junctions functionally couple the smooth muscle cells so that contraction of bundles occurs synchronously. The interstitial cells of Cajal (ICCs) are a specialized group of cells in the intestinal wall that are involved in the transmission of information from enteric neurons to smooth muscle cells (Fig. 28-12). It is also thought that ICCs are "pacemaker" cells that have the capacity to generate the basic electrical rhythm, or "slow wave" activity,
that is a consistent feature of GI smooth muscle (Fig. 28-13).
|
| Electrophysiology of Gastrointestinal Smooth Muscle
|
| Figure 28-12 Diagrammatic representation of the interstitial cells of Cajal network in the smooth muscle wall of the GI tract. |
| Figure 28-13 Excitation coupling in GI smooth muscle. The slow wave will initiate a contraction in smooth muscle when it reaches a threshold amplitude. The amplitude of the slow wave is altered by release of neurotransmitters from enteric neurons. |
| page 512 |  | | page 513 |
| The resting membrane potential of GI smooth muscle varies characteristically with time-the basic electrical rhythm, or slow wave. The frequency of slow waves is 3 to 5 per minute in the stomach and about 12 to 20 per minute in the small intestine; it decreases to 6 to 8 per minute in the colon. The frequency of the slow
wave is set by a pacemaker region in the different regions of the GI tract (Fig. 28-13). The slow wave of that particular region of the GI tract will entrain to the fastest frequency of the slow wave as it is transmitted through the adjacent muscle bundles via gap junctions. Slow waves are thought to be generated by interstitial cells (ICCs). These cells are located in a thin layer between the longitudinal and circular layers of the muscularis externa and in other places in the wall of the GI tract. Interstitial cells have properties of both fibroblasts and smooth muscle cells. Their long processes form gap junctions with the longitudinal and circular smooth muscle cells; the gap junctions enable the slow waves to be conducted rapidly to both muscle layers. Because gap junctions electrically and chemically couple the smooth muscle cells of both longitudinal and circular layers, the slow wave spreads throughout the smooth muscle of each segment of the GI tract.
|
| The amplitude and, to a lesser extent, the frequency of the slow wave can be modulated by the activity of intrinsic and extrinsic nerves and by hormones and paracrine substances. If the depolarization of the slow wave exceeds the threshold, a train of action potentials may be triggered during the peak of the slow wave. Action potentials in GI smooth muscle are more prolonged (10 to 20 msec) than those in skeletal muscle and have little or no overshoot. The rising phase of the action potential is caused by flow of ions through channels that conduct both Ca++ and Na+ and are relatively slow to open. The Ca++ that enters the cell during the action potential helps initiate contraction. The extent of depolarization of the cells and the frequency of action potentials are enhanced by some hormones and paracrine agonists and by neurotransmitters from excitatory enteric nerve endings (e.g., acetylcholine and substance P). Inhibitory hormones and neuroeffector substances (e.g., vasoactive intestinal polypeptide and nitric oxide) hyperpolarize the smooth muscle cells and may diminish or abolish action potential spikes.
|
| Slow waves that are not accompanied by action potentials elicit little or no contraction of the smooth muscle cells. Much stronger contractions are evoked by the presence of action potentials. The greater the number of action potentials that occur at the peak of a slow wave, the more intense the contraction of the smooth muscle. Because smooth muscle cells contract rather slowly (about a 10th as fast as skeletal muscle cells), the individual contractions caused by each action potential in a train do not cause distinct twitches; rather, they sum temporally to produce a smoothly increasing level of tension.
|
| Between the trains of action potentials, the tension developed by GI smooth muscle falls, but not to zero. This nonzero resting, or baseline, tension of smooth muscle is called tone. The tone of GI smooth muscle is altered by neuroeffectors, hormones, paracrine substances, and drugs and is important in the sphincters and also in regions where storage of contents is important, such as the stomach and the colon.
|
| Specialized Patterns of Motility
|
| Peristalsis is a moving ring of contraction that propels material along the GI tract. It involves neurally mediated contraction and relaxation of both muscle layers. Peristalsis occurs in the pharynx, esophagus, gastric antrum, and the small and large intestine.
|
| Segmental contractions produce narrow areas of contracted segments between relaxed segments. These movements allow mixing of the luminal contents with GI tract secretions and increase exposure to the mucosal surfaces where absorption occurs. Segmentation occurs predominantly in the small and large intestine.
|
| There are also characteristic pathological patterns of motility. During spasm, maximal contractile activity occurs continuously in a dysregulated manner. In ileus, contractile activity is markedly decreased or absent; it often results from irritation of the peritoneum, such as occurs in surgery, peritonitis, and pancreatitis.
|
| Functional Anatomy of the Stomach
|
| As discussed, the stomach is divided into two functional regions-proximal and distal, with sphincters at either end. The LES and cardia (defined as the region of the stomach immediately surrounding the LES) have important functions. Relaxation of the LES and cardia allows entry of food from the esophagus into the stomach and the release of gas, called belching. By maintaining tone, reflux of contents from the stomach back into the esophagus is prevented.
|
| The proximal part of the stomach (the fundus together with the corpus or body) produces slow changes in tone compatible with its reservoir function. It is important for receiving and storing food and for mixing the contents with gastric juice (Table 28-3). Generation of tone in the proximal portion of the stomach is also an important driving force in the regulation of gastric emptying. Low tone and consequently low intragastric pressure are associated with delayed or slow gastric emptying, and an increase in tone in this region is required for gastric emptying to occur.
|
|
Table 28-3.
The Stomach Alters the Physical and Chemical Characteristics of the Meal |
| Input | Output |
| Bolus | Emulsion, suspension (particles <2 mm) |
| Triglyceride | Triglyceride plus small amounts of 2-monoglycerides and free fatty acids |
| Protein | Protein plus small amounts of peptides and amino acids |
| Starch | Starch plus oligosaccharides |
| Water, ions | Addition of large amounts of water and ions of low pH |
| page 513 |  | | page 514 |
| The distal part of the stomach is important in the mixing of gastric contents and for propulsion through
the pylorus and into the duodenum. The muscle layers in the region of the gastric antrum are much thicker than in the more proximal regions of the stomach, and thus the antrum is capable of producing strong phasic contractions. Contractions, initiated by the slow wave, begin in the midportion of the stomach and move toward the pylorus. The strength of these contractions varies during the postprandial period. In the gastric phase of the meal, the pylorus is usually closed, and these antral contractions serve to mix the gastric contents and reduce the size of solid particles (grinding). However, eventually these antral contractions are also important in emptying the stomach of its contents.
|
| The pyloric sphincter is the gastroduodenal junction and is defined as an area of thickened circular muscle. This is a region of high pressure generated by tonic smooth muscle contraction. It is important in regulating gastric emptying.
|
| Control of Gastric Motility in the Gastric Phase
|
| Gastric motility is highly regulated and coordinated to perform the functions of storage and mixing. Regulation of emptying of contents into the small intestine, an important part of gastric motor function, will be considered in detail in the discussion of the duodenal phase of the meal because the controls are generated in the duodenum.
|
| The stimuli regulating gastric motor function that result from the presence of the meal in the stomach are both mechanical and chemical and include distention and the presence of products of protein digestion (amino acids and small peptides). The pathways regulating these processes are predominantly neural and consist of vagovagal reflexes initiated by extrinsic vagal afferent fibers that terminate in the muscle and mucosa. Mucosal afferents respond to chemical stimuli, and mechanosensitive afferents respond to distention and contraction of smooth muscle. This afferent stimulation results in reflex activation of vagal efferent (parasympathetic) outflow and activation of enteric neurons that innervate the smooth muscle. Activation of enteric neurons produces both inhibitory and excitatory effects on gastric smooth muscle; these effects vary, depending on the region of the stomach. Thus, distention of the gastric wall results in inhibition of smooth muscle in the proximal portion of the stomach and subsequent reflex accommodation, which allows entry and storage of the meal to occur with minimal increase in intragastric pressure.
|
|
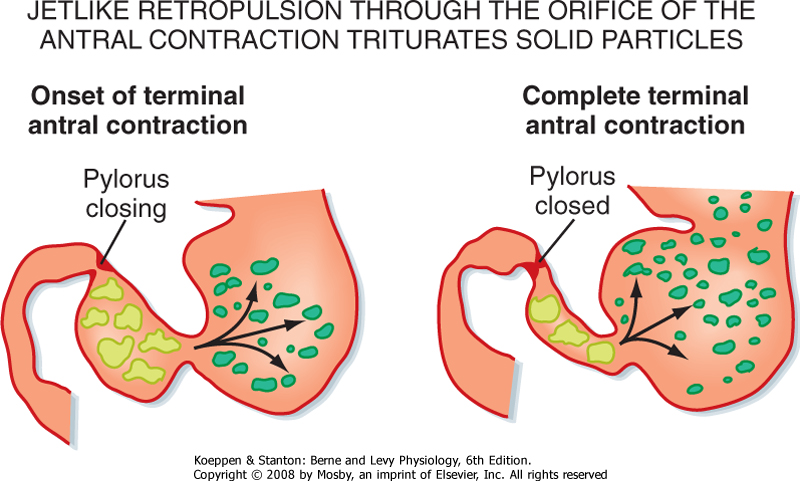
| Figure 28-14 Coordinated activity in the smooth muscle of the proximal and distal portions of the stomach and the pyloric sphincter results in mixing and grinding in the gastric antrum. The peristaltic wave moves down the gastric body and antrum toward the pylorus. If the pylorus is closed, the contents of the gastric antrum are retropulsed back into the more proximal part of the stomach. This pattern of motility results in grinding and mixing of the food with secretions from the gastric wall and eventually leads to a reduction in particle size and the presence of digestive products that will empty into the duodenum. |
| In contrast, the predominant motor pattern of the distal part of the stomach in the gastric phase of the meal is activation of smooth muscle to produce and strengthen the antral contractions. The rate of antral contractions is set by the gastric pacemaker; however, the magnitude of the contractions is regulated by the release of neurotransmitters from enteric neurons, including substance P and acetylcholine, which increase the level of depolarization of the smooth
muscle and therefore produce stronger contractions. In this phase of the meal the pylorus is mostly closed. Thus, antral contractions will tend to move the contents toward the pylorus; however, because the pylorus is closed, the contents will be returned to the more proximal part of the stomach. In this way, the gastric contents will be mixed. In addition, antral contractions can occlude the lumen, and thus larger particles will be dispersed, a process referred to as grinding (Fig. 28-14).
|
| page 514 |  | | page 515 |
- The main functions of the stomach are storage and initiation of protein digestion.
- Regulation of gastric function is driven by extrinsic and intrinsic neural pathways, together with key humoral (gastrin) and paracrine (histamine) mediators.
- The key secretions from the stomach are acid and pepsinogen, which together begin protein digestion.
- H+ is secreted across the apical plasma membrane of parietal cells via H+,K+-ATPase, the proton pump.
- The only secretion by the stomach that is required is intrinsic factor, which is involved in the absorption of vitamin B12.
- The gastric epithelium secretes HCO3- and mucus to form a gel-like mucosal barrier that protects it against the acidic and peptic luminal contents.
- The smooth muscle of the gut wall undergoes cyclic changes in membrane potential, termed the basic electrical rhythm or the slow wave.
- The interstitial cells of Cajal are pacemakers in the gut wall, and they set the frequency of the slow wave.
- The proximal part of the stomach undergoes a slow change in tone compatible with its storage function.
- The distal part of the stomach undergoes phasic contractions that can vary considerably in strength.
- Gastric emptying is regulated by vagovagal reflexes.
|
 |
|








 to protect the gastric mucosa
to protect the gastric mucosa