| 30 The Colonic Phase of the Integrated Response to a Meal
|
| OVERVIEW OF THE LARGE INTESTINE
|
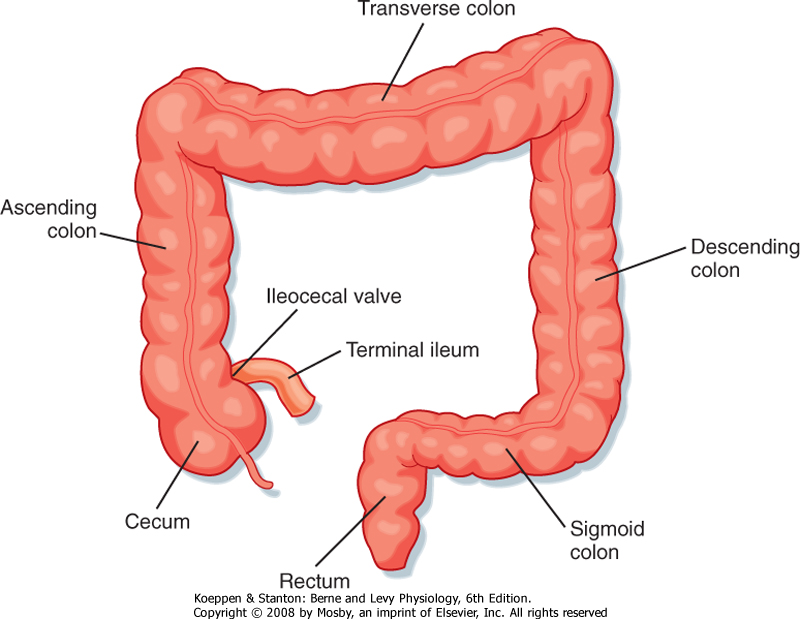
| The most distal segment of the gastrointestinal tract is called the large intestine, which is composed of the cecum; ascending, transverse, and descending portions of the colon; the rectum; and the anus (Fig. 30-1). The primary functions of the large intestine are to digest and absorb components of the meal that cannot be digested or absorbed more proximally, reabsorb the remaining fluid that was used during movement of the meal along the gastrointestinal tract, and store the waste products of the meal until they can conveniently be eliminated from the body. In fulfilling these functions, the large intestine uses characteristic motility patterns and expresses transport mechanisms that drive the absorption of fluid, electrolytes, and other solutes from the stool. The large intestine also contains a unique biological ecosystem consisting of many trillions of so-called commensal bacteria that engage in a life-long symbiotic relationship with their human host. These bacteria can metabolize components of the meal that are not digested by host enzymes and make their products available to the body via a process known as fermentation. Colonic bacteria also metabolize other endogenous substances such as bile acids and bilirubin, thereby influencing their disposition. There is emerging evidence that the colonic flora is critically involved in promoting development of the normal colonic epithelium and in stimulating its differentiated functions. In addition, these bacteria detoxify xenobiotics (substances originating outside the body, such as drugs) and protect the colonic epithelium from infection by invasive pathogens. Finally, the colon is both the recipient and the source of signals that allow it to communicate with other gastrointestinal segments to optimally integrate function. For example, when the stomach is filled with freshly masticated food, the presence of the meal triggers a long reflex arc that results in increased colonic motility (the gastrocolic reflex) and eventually evacuation of the colonic contents to make way for the residues of the next meal. Similarly, the presence of luminal contents in the colon causes the release of both endocrine and neurocrine mediators that slow propulsive motility and decrease electrolyte secretion in the small intestine. This negative-feedback mechanism matches the rate of delivery of colonic contents to the segment's capacity to process and absorb the useful components. Details of the signals that mediate this crosstalk between the colon and other components of the gastrointestinal system are reviewed in the next section.
|
| Signals That Regulate Colonic Function
|
| The colon is regulated primarily, though not exclusively, by neural pathways. Colonic motility is influenced by local reflexes that are generated by filling of the lumen, thereby initiating distention and the activation of stretch receptors. These regulatory pathways exclusively involve the enteric nervous system. Local reflexes, triggered by distortion of the colonic epithelium and produced, for example, by the passage of a bolus of fecal material, stimulate short bursts of Cl- and fluid secretion mediated by 5-hydroxytryptamine (5-HT) from enteroendocrine cells and acetylcholine from enteric secretomotor nerves. On the other hand, colonic function and motility responses in particular are also regulated by long reflex arcs originating more proximally in the gastrointestinal tract or in other body systems. One example of such a reflex is the gastrocolic reflex. Distention of the stomach activates a generalized increase in colonic motility and mass movement of fecal material, as described in more detail later. This reflex has both chemosensitive and mechanosensitive components at its site of origin and involves the release of 5-HT and acetylcholine. Similarly, the orthocolic reflex is activated on rising from bed and promotes a morning urge to defecate in many individuals.
|
| page 533 |  | | page 534 |
| Figure 30-1 Major anatomic subdivisions of the colon. |
| The colon is relatively poorly supplied with cells that release bioactive peptides and other regulatory factors. Exceptions are enterochromaffin cells, which release 5-HT, and cells that synthesize peptide YY, so named because its sequence contains two adjacent tyrosine residues (Y is the single letter code for amino acids). Peptide YY is synthesized by enteroendocrine cells localized in the terminal ileum and colon and is released in response to lipid in the lumen. It decreases gastric emptying and intestinal propulsive motility. Peptide YY also reduces Cl- and thus fluid secretion by intestinal epithelial cells. Thus, peptide YY has been characterized as an "ileal brake" in that it is released if nutrients, especially fat, are not absorbed by the time that the meal reaches the terminal ileum and proximal part of the colon. By reducing propulsion of the intestinal contents, in part by limiting their fluidity and distention-induced motility, peptide YY
provides more time for the meal to be retained in the small intestine, where its constituent nutrients can be digested and absorbed.
|
| Patterns of Colonic Motility
|
| To appreciate colonic motility, the functional anatomy of the colonic musculature will be reviewed first, followed by a discussion of the regulation of colonic motility.
|
| Functional Anatomy of the Colonic Musculature
|
| As in other segments of the intestine, the colon consists of functional layers with a columnar epithelium most closely opposed to the lumen, which is then underlaid by the lamina propria, serosa, and muscle layers. Similarly, the colonic mucosa is surrounded by continuous layers of circular muscle that can occlude the lumen. Indeed, at intervals, the circular muscle contracts to divide the colon into segments called haustra. These haustra are readily appreciated if the colon is viewed at laparotomy or by x-ray imaging as shown in Figure 30-2. The arrangement of the majority of the longitudinal muscle fibers, however, is distinct from that in the small intestine. Three nonoverlapping bands of longitudinal muscle, known as the taeniae coli, extend along the length of the colon.
|
| Figure 30-2 Radiograph showing a prominent haustral pattern in the colon of a normal individual. (From Keats TE: An Atlas of Normal Roentgen Variants, 2nd ed. St Louis, Mosby-Year Book, 1979.) |
| page 534 |  | | page 535 |
| Although the circular and longitudinal muscle layers of the colon are electrically coupled, this process is less efficient than in the small intestine. Thus, propulsive motility in the colon is less effective than in the small intestine. Activity of the enteric nervous system also provides for the segmenting contractions that form the haustra. Contents can be moved back and forward between haustra, which is a means of retarding passage of the colonic contents and maximizing their contact time with the epithelium. In contrast, when rapid propulsion is called for, the contractions
forming the haustra relax, and the contour of the colon is smoothened.
|
| Hirschsprung's disease is a condition in which a segment of the colon remains permanently contracted and results in obstruction. It is typically diagnosed in infancy and affects up to 1 in 5000 live births in the United States. The basis of the disease is failure of the enteric nervous system to develop normally during fetal life. During organogenesis, cells destined to become enteric neurons migrate out from the neural crest and populate the gut sequentially from mouth to anus. In some individuals, this migration terminates prematurely because of abnormalities in the mechanisms that would otherwise drive this process. Mutations in glial-derived neurotrophic factor and endothelin III, as well as in their receptors, have been described in individuals suffering from this disease, and the affected segment completely lacks the plexuses of the enteric nervous system and associated ganglia. A relative deficiency of interstitial cells of Cajal is also seen in the affected segment, and overall control of motility is markedly impaired. In most individuals, the symptoms can be completely alleviated by surgical excision of the affected segment. |
 |
|
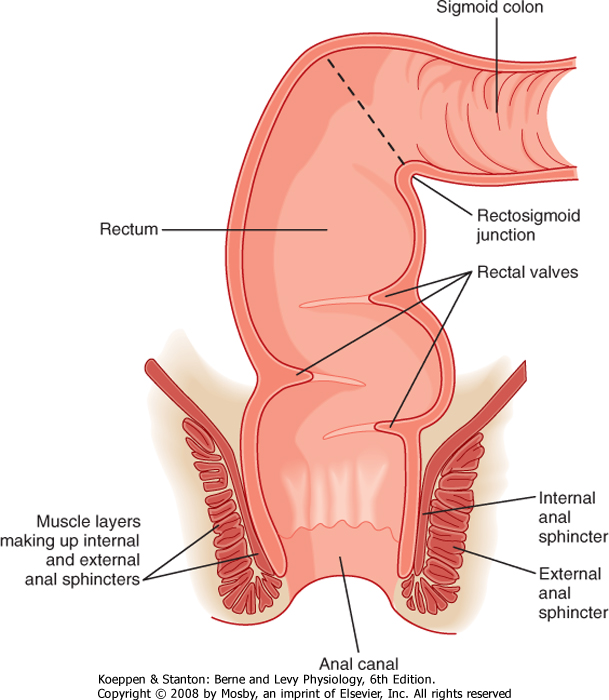
| Figure 30-3 Anatomy of the rectum and anal canal. |
| The colon terminates in the rectum, which is joined to the colon at an acute angle (the rectosigmoid junction) (Fig. 30-3). The rectum lacks circular muscle and
is surrounded only by longitudinal muscle fibers. It is a reservoir wherein feces can be stored before defecation. Muscular contractions also form functional "valves" in the rectum that retard the movement of feces and are important in delaying the loss of feces until it is convenient, at least in adults. The rectum, in turn, joins the anal canal, distinguished by the fact that it is surrounded not only by smooth muscle but also by striated (skeletal) muscle. The combination of these muscle layers functionally accounts for two key sphincters that control the evacuation of solid waste and flatus from the body. The internal anal sphincter is composed of a thickened band of circular muscle, whereas the external anal sphincter is made up of three different striated muscle structures in the pelvic cavity that wrap around the anal canal. These latter muscles are distinctive because they maintain a significant level of basal tone and can be contracted further either voluntarily or reflexively when abdominal pressure increases abruptly (such as when lifting a heavy object).
|
| page 535 |  | | page 536 |
| Contraction of the smooth muscle layers in the proximal part of the colon is stimulated by vagal input, as well as by the enteric nervous system. On the other hand, the remainder of the colon is innervated by the pelvic nerves, which also control the caliber of the internal anal sphincter. Voluntary input from the spinal cord via branches of the pudendal nerves regulates contraction of the external anal sphincter and muscles of the pelvic floor. The ability to control these structures is learned during toilet training. This voluntary
control distinguishes the anal canal from most of the gastrointestinal system, with the exception of the striated muscle in the esophagus that regulates swallowing.
|
| Colonic Motility Responses
|
| Consistent with its primary function, the two predominant motility patterns of the large intestine are directed not to propulsion of the colonic contents but rather to mixing of the contents and retarding their movement, thereby providing them with ample time in contact with the epithelium. Two distinctive forms of colonic motility have been identified. The first is referred to as short-duration contractions, which are designed to provide for mixing. These contractions originate in the circular muscle and are stationary pressure waves that persist for approximately 8 seconds on average. Long-duration contractions, in contrast, are produced by the taeniae coli, last for 20 to 60 seconds, and may propagate over short distances. Notably, however, propagation may move orally as well as aborally, particularly in the more proximal segments of the colon. Both these motility patterns are thought to originate largely in response to local conditions, such as distention. Note that the basal electrical rhythm that governs the rate and origination sites of smooth muscle contraction in the small intestine does not traverse the ileocecal valve to continue into the colon.
|
| On the other hand, probably as a result of both local influences and long reflex arcs, approximately 10 times per day in healthy individuals the colon engages in a motility pattern that is of high intensity and sweeps along the length of the large intestine from the cecum to the rectum. Such contractions, which are labeled "high-amplitude propagating contractions," move exclusively in an aboral direction and are designed to clear the colon of its contents. However, although such a motility pattern can clearly be associated with defecation, it does not necessarily result in defecation for reasons discussed later.
|
| It is also important to note that there is considerable variability among individuals with respect to the rate at which colonic contents are transported from the cecum to the rectum. Although small intestinal transit times are relatively constant in healthy adults, the contents may be retained in the large intestine anywhere from hours to days without signifying dysfunction. This likewise accounts for significant variation among individuals in their normal patterns of defecation and mandates careful elicitation of a patient's history before diagnosing bowel dysfunction.
|
| Transport Mechanisms in the Colon
|
| Irritable bowel syndrome is the name given to a heterogeneous collection of disorders whose sufferers complain of diarrhea, constipation, or alternating patterns of both, often with accompanying pain and distention. The precise cause or causes of these disorders are still not fully understood but may involve, in part, a condition of visceral hypersensitivity in which the individual perceives normal signals originating from the bowel (such as in response to distention) as painful. This hypersensitivity may be at the level of the enteric or central nervous system (or both) and can be triggered by a variety of factors such as previous infections, childhood abuse, or psychiatric disorders. Most treatments focus on symptomatic relief, but there is the promise of more effective therapies as we learn more about the underlying causes of the condition. Treatment of patients with irritable bowel disorders, which are often refractory to therapy, forms a major part of the practice of many gastroenterologists in the community. |
 |
| The rapid turnover of the colonic epithelium, as well as frequent/prolonged exposure to bacterially synthesized or environmental toxins, or both, makes the large intestine especially vulnerable to malignancy. Colon cancer is second in prevalence only to lung cancer in men in the United States and third behind lung and breast cancer in women. With the decreased incidence of cigarette smoking, colon cancer may assume even greater significance. Colon cancer arises when normal genetic controls on the rate of epithelial proliferation are subverted; initially, this leads to growth of a polyp and, eventually, if not removed, to an invasive tumor that may metastasize to other parts of the body. Colon cancer can be subdivided according to the basic nature of the underlying molecular defect, which can include overexpression of growth stimulatory factors or a mutation that prevents the cells from responding to factors that would normally be growth suppressive. However, colon cancer mortality can be reduced very substantially by early detection and removal of polyps with malignant potential. This has driven current guidelines for increased screening of even asymptomatic middle-aged individuals for colonic abnormalities via colonoscopy (in which a flexible fiberoptic tube is inserted into the colon to inspect its interior), screening for the presence of so-called occult (or hidden) blood in the stool derived from a bleeding polyp or tumor, or noninvasive imaging techniques such as computed tomography scans. |
 |
| page 536 |  | | page 537 |
| The surface cells are renewed from stem cells located at the base of the crypts; the stem cells give rise to migrating cells that gradually acquire differentiated properties as they move to the surface. The colonic epithelium turns over rapidly even in health, thus limiting the accumulation of genetic damage that might otherwise be caused by exposure to toxins in the lumen. However, the rapid turnover also increases the risk for malignancy. The major role of the colonic
epithelium is to either absorb or secrete electrolytes and water rather than nutrients. Secretion, which is confined to the crypts, maintains the sterility of the crypts, which might otherwise become stagnant. However, the colonic epithelium absorbs short-chain
fatty acids salvaged from nonabsorbed carbohydrates by colonic bacteria. Indeed, one such short-chain fatty acid, butyrate, is a critical energy source for colonocytes. A reduction in butyrate levels in the lumen (as a result of changes in colonic flora caused by the administration of broad-spectrum antibiotics) may induce epithelial dysfunction.
|
| The colon receives 2 L of fluid each day and absorbs 1.8 L, thus leaving 200 mL of fluid to be lost in stool. The colon has a considerable reserve capacity for fluid absorption and can absorb up to three times its normal fluid load without loss of excessive fluid into stool. Therefore, any illness that results in the stimulation of active fluid secretion in the small intestine will cause diarrhea only when the reserve capacity of 4 to 6 L is exceeded.
|
| Absorption and secretion of water by the colon are passive processes driven by the absorption or secretion of electrolytes and other solutes. Quantitatively, fluid absorption by the colon is driven by three transport processes. The first is electroneutral NaCl absorption, which is mediated by the same mechanism that drives NaCl absorption in the intestine (see Fig. 29-17). NaCl absorption is stimulated by various growth factors, such as epidermal growth factor, and is inhibited by hormones and neurotransmitters that increase levels of cAMP in colonic surface epithelial cells.
|
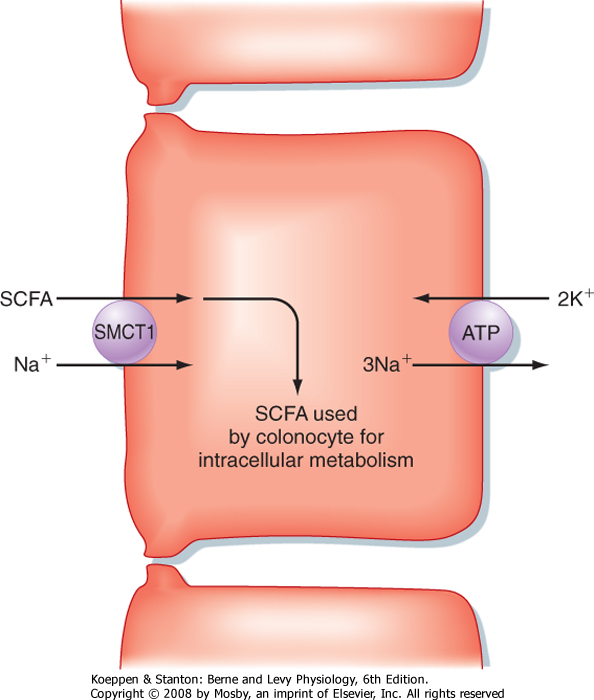
| The second transport process that drives fluid absorption in the colon is the absorption of short-chain fatty acids, including acetate, propionate, and butyrate. These molecules are absorbed from the lumen by surface (and perhaps crypt) epithelial cells in an Na+-dependent fashion by a family of symporters related to the Na+-glucose symporter in the small intestine known as sodium-monocarboxylate transporters (SMCTs). Uptake of short-chain fatty acids by SMCTs located in the apical plasma membrane is driven by the low intracellular [Na+] established by the basolateral Na+,K+-ATPase (Fig. 30-4). These short-chain fatty acids are used for energy by colonocytes. In addition, butyrate regulates the expression of specific genes in colonic epithelial cells and may suppress the development of a malignant phenotype. Expression of SMCT1 (also identified as SLC5A8) is reduced in some colon cancers, thereby leading to a reduction in butyrate uptake, which may contribute to malignant transformation.
|
|
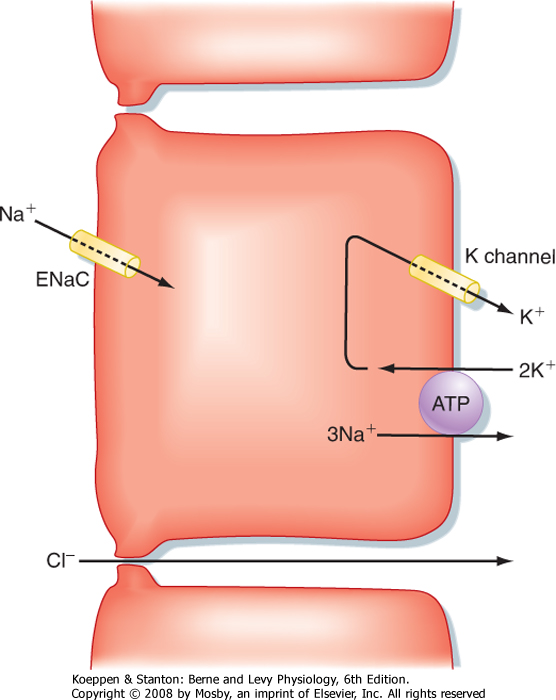
| Figure 30-4 Mechanism of short-chain fatty acid (SCFA) uptake by colonocytes. |
| Figure 30-5 Electrogenic Na+ absorption in the colon. |
| page 537 |  | | page 538 |
| The third absorptive process of major significance in the colon is the absorption of Na+ (Fig. 30-5). This transport process is predominantly localized to the distal part of the colon and is driven by the epithelial Na+ channel ENaC, which is also involved in reabsorption of Na+ in the kidney. When the channel is opened in response to activation by neurotransmitters or hormones, or both, Na+ flows into the colonocyte cytosol and is then transported across the basolateral membrane by Na+,K+-ATPase. Water and Cl- ions follow passively via the intercellular tight junctions to maintain electrical neutrality. This mode of Na+ absorption is the last line of defense to prevent excessive loss of water in stool, given its strategic location in the distal part of the colon. Indeed, patients suffering from bowel
inflammation often show markedly diminished expression of ENaC, perhaps accounting for their diarrheal symptoms. We also know that expression of ENaC can be acutely regulated in response to whole-body Na+ balance. Thus, in situations of reduced Na+ intake, the hormone aldosterone increases ENaC expression in
both the colon and kidney, thereby promoting retention of Na+.
|
| Adequate hydration of the colonic contents is determined by the balance between water absorption and secretion. Fluid secretion in the colon is driven by Cl- ion secretion, by the same mechanism driving fluid secretion in the small intestine, and is subject to the same regulation (see Fig. 29-18). Indeed, some cases of constipation may reflect abnormalities in epithelial transport, and constipation that results from abnormally slow motility can be treated by agents that stimulate Cl- secretion. Conversely, excessive Cl- secretion can be one mechanism underlying diarrhea.
|
| The remnants of the meal entering the colon interact with a vast assortment of bacteria. This enteric bacterial ecosystem is established shortly after birth and remains remarkably stable unless perturbed by antibiotics or the introduction of an aggressive pathogen. The enteric bacterial ecosystem contributes to gastrointestinal physiology in a surprising number of ways. Indeed, the large intestine (and to a lesser extent the distal portion of the small intestine) is a highly unusual organ in that it maintains a symbiotic relationship with the bacterial ecosystem, whereas other body compartments are largely sterile.
|
| Diarrheal diseases are a major cause of infant mortality worldwide and are usually the result of inadequate access to clean food and water. Even in developed countries, diarrheal diseases cause substantial suffering and occasional, well-publicized deaths and carry a substantial economic burden because of their prevalence. Infectious diarrhea is caused by a number of organisms, with several (such as cholera or pathogenic strains of Escherichia coli) capable of elaborating toxins that trigger excessive increases in active Cl- secretion by small and large intestinal epithelial cells. Diarrhea can also result when nutrients are not appropriately digested and absorbed in the small intestine (e.g., lactose intolerance) or as a result of colonic inflammation. In most diarrheal diseases, colonic NaCl and Na+ absorption are down-regulated at the same time that Cl- secretion may be stimulated, thus further worsening fluid loss. On the other hand, nutrient-linked Na+ absorptive processes typically remain intact. This provides the rationale for the effectiveness of so-called oral rehydration solutions, which are prepackaged mixtures of salt and glucose. Uptake of Na+ and glucose from these solutions, mediated by SGLT1 (see Chapter 29), drives water back into the body to balance osmotic forces. These solutions save lives in areas where diarrhea is prevalent and the ability to rehydrate patients with sterile intravenous solutions is limited or absent. |
 |
| The colonic microflora is not essential to life because animals raised in germ-free conditions apparently
develop normally and are able to reproduce. However, in such animals the mucosal immune system is immature, and intestinal epithelial cells differentiate more slowly. However, the colonic flora provides benefits to the host in that the constituent bacteria are capable of performing metabolic reactions that do not take place in mammalian cells. Bacterial enzymes act on both endogenous and exogenous substrates. They form secondary bile acids and deconjugate any bile acids that have escaped uptake in the terminal ileum so that they can be reabsorbed. They convert bilirubin into urobilinogen (see Chapter 31) and salvage nutrients that are resistant to pancreatic and brush border hydrolases, such as dietary fiber. A summary of the metabolic contributions of the colonic microflora is provided in Table 30-1. Bacterial metabolism can also be exploited for pharmacological purposes. A drug targeted to the colon, for example, can be conjugated in such a way that it will become bioavailable only after it is acted on by bacterial enzymes. Bacterial enzymes may also detoxify some dietary carcinogens, but equally, they may generate toxic or carcinogenic compounds from dietary substrates.
|
| A toxin known as heat-stable toxin of E. coli, or STa, is a major causative agent of traveler's diarrhea, which can be contracted by the consumption of infected food or water. This toxin binds to a receptor on the apical surface of intestinal epithelial cells known as guanylyl cyclase C (GC-C). In turn, this enzyme generates large quantities of intracellular cGMP that trigger increased Cl- secretion via activation of the cystic fibrosis transmembrane conductance regulator (CFTR) Cl- channel. However, one could, of course, question, why humans express a receptor for this toxin in a site that would be accessible to luminal bacteria and their products. Indeed, this led to the hypothesis that there is a native ligand for GC-C that could play a physiological role. This hypothesis led to purification and identification of guanylin, a hormone synthesized in the intestine. Together with a related molecule, uroguanylin, secreted by the kidney, guanylin is an important regulator of salt and water homeostasis in the body. STa has structural similarities to guanylin, but it has modifications that permit it to persist in the intestinal lumen for prolonged periods. This is an example of molecular mimicry in which a bacterial product hijacks a receptor and associated signaling for its own purposes (presumably to propagate the toxin-producing bacteria to additional hosts). |
 |
| page 538 |  | | page 539 |
|
Table 30-1.
Metabolic Effects of Enteric Bacteria |
| Substrate | Enzymes | Products | Disposition |
| Endogenous Substrates |
| Urea | Urease | Ammonia | Passive absorption or excretion as ammonium |
| Bilirubin | Reductases | Urobilinogen
Stercobilins | Passive reabsorption
Excreted |
| Primary bile acids | Dehydroxylases | Secondary bile acids | Passive reabsorption |
| Conjugated bile acids (primary or secondary) | Deconjugases | Unconjugated bile acids | Passive reabsorption |
| Exogenous Substrates |
| Fiber | Glycosidases | Short-chain fatty acids
Hydrogen, CO2, and methane | Active absorption
Excreted in breath or flatus |
| Amino acids | Decarboxylases and deaminases | Ammonia and bicarbonate | Reabsorbed or excreted (ammonia) as ammonium |
| Cysteine, methionine | Sulfatases | Hydrogen sulfide | Excreted in flatus |
|
Adapted from Barrett KE: Gastrointestinal Physiology. New York, McGraw-Hill, 2006.
|
| Commensal microorganisms also play a critical role in limiting the growth or invasion (or both) of pathogenic microorganisms. They fulfill this antimicrobial role via a number of different mechanisms-by synthesizing and secreting compounds that inhibit the growth of competitor organisms or that are microbicidal, by
functioning as a physical barrier to prevent attachment of pathogens and their subsequent entry into colonic epithelial cells, and by triggering patterns of gene expression in the epithelium that counteract the adverse effects of pathogens on epithelial function. These mechanisms provide a basis for understanding why patients who have received broad-spectrum antibiotics, which temporarily disrupt the colonic microflora, are susceptible to overgrowth of pathogenic organisms and associated intestinal and systemic infections. They may also shed light on the efficacy of probiotics, commensal bacteria selected for their resistance to gastric acid and proteolysis that are intentionally ingested to prevent or treat a variety of digestive disorders.
|
| The colonic microflora is also notable for its contribution to the formation of intestinal gas. Although large volumes of air may be swallowed in conjunction with meals, the majority of this gas returns up the esophagus via belching. However, during fermentation of unabsorbed dietary components, the microflora generates large volumes of nitrogen, hydrogen, and carbon dioxide. Approximately 1 L of these nonodorous gases is excreted on a daily basis via the anus in all individuals, even those who do not complain of flatulence. Some individuals may generate appreciable concentrations of methane. Trace amounts of odorous compounds are also present, such as hydrogen sulfide, indole, and skatole.
|
| The final stage in the journey taken by a meal after its ingestion is expulsion of its indigestible residues from the body in the process known as defecation. The feces also contain the remnants of dead bacteria; dead and dying epithelial cells that have been desquamated from the lining of the intestine; biliary metabolites specifically targeted for excretion, such as conjugates of xenobiotics (see Chapter 31); and a small amount of water. In health, the stool contains little, if any useful nutrients. The presence of such nutrients in stool, particularly lipid (known as steatorrhea), signifies maldigestion, malabsorption, or both. Fat in the stool is a sensitive marker of small intestinal dysfunction because it is poorly used by the colonic microflora, but loss of carbohydrate and protein in stool can also be seen if the underlying condition worsens.
|
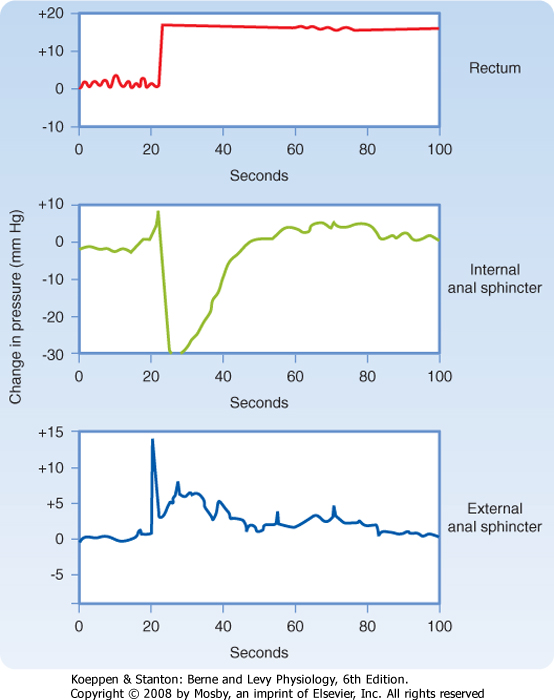
| The process of defecation requires coordinated action of the smooth and striated muscle layers in the rectum and anus, as well as surrounding structures such as the pelvic floor muscles. During the mass movement of feces produced by high-amplitude propagating contractions, the rectum fills with fecal material. Expulsion of this material from the body is controlled by the internal and external anal sphincters, which contribute approximately 70% to 80% and 20% to 30% of anal tone at rest, respectively. Filling of the rectum causes relaxation of the internal anal sphincter via the release of vasoactive intestinal polypeptide and the generation of nitric oxide. Relaxation of the inner sphincter permits the anal sampling mechanism, which can distinguish whether the rectal contents are solid, liquid, or gaseous in nature. After toilet training, sensory nerve endings in the anal mucosa then generate reflexes that initiate appropriate activity of the external sphincter to either retain the rectal contents or permit voluntary expulsion (e.g., of flatus). If defecation is not convenient, the external sphincter contracts to prevent the loss of stool. Then, with time, the rectum accommodates to its new volume, the internal anal sphincter contracts again, and the external anal sphincter relaxes (Fig. 30-6).
|
| page 539 |  | | page 540 |
| Figure 30-6 Responses of the internal and external anal sphincters to prolonged distention of the rectum. Note that the responses of the sphincters are transient because of accommodation. (Redrawn from Shuster MM et al: Bull Johns Hopkins Hosp 116:79, 1965.) |
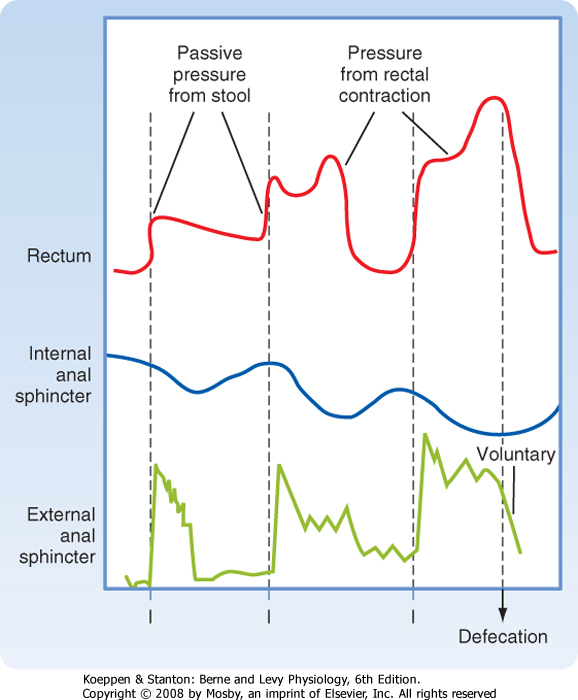
| Figure 30-7 Motility of the rectum and anal sphincters in response to rectal filling and during defecation. Note that filling of the rectum causes an initial decrease in internal sphincter tone that is counterbalanced by contraction of the external sphincter. The internal sphincter then accommodates to the new rectal volume, thereby allowing the external sphincter to relax. Finally, defecation occurs when the external anal sphincter is relaxed voluntarily. (Data from Chang EB et al: Gastrointestinal, Hepatobiliary and Nutritional Physiology. Philadelphia, Lippincott-Raven, 1996.) |
| page 540 |  | | page 541 |
| When defecation is desired, on the other hand, adoption of a sitting or squatting position alters the relative orientation of the intestine and surrounding muscular structures by straightening the path for the exit of either solid or liquid feces. Relaxation of the puborectalis muscle likewise increases the rectoanal angle. After voluntary relaxation of the external anal sphincter, rectal contractions move the fecal material out of the body, sometimes followed by additional mass movements of feces from more proximal segments of the colon (Fig. 30-7). Evacuation is assisted by simultaneous contraction of muscles that increase abdominal pressure, such as the diaphragm. The voluntary expulsion of flatus, on the other hand, involves
a similar sequence of events, except that there is no relaxation of the puborectalis muscle. This permits flatus to be squeezed past the acute rectoanal angle while retaining fecal material.
|
| Cooperative activity of the external anal sphincter, puborectalis muscle, and sensory nerve endings in the anal canal is required to delay defecation until it is appropriate, even if the rectum is acutely distended with stool or intraabdominal pressure rises sharply. This explains why incontinence can develop in individuals in whom the integrity of such structures has been compromised, such as after trauma, surgical or obstetrical injuries, prolapse of the rectum, or neuropathic diseases such as long-standing diabetes. Surgical intervention may be necessary to correct muscle abnormalities in patients with the distressing condition of fecal incontinence, although many can be helped to increase the tone of their external anal sphincter with the use of biofeedback exercises.
|
- The final segment of the intestine through which the meal traverses is the large intestine, which is composed of the cecum, colon, rectum, and anus. The primary role of the large intestine is to reclaim water used during the process of digestion and absorption and to store the residues of the meal until defecation is socially convenient.
- Colonic motility primarily serves to mix and delay passage of the luminal contents, other than during periodic large-amplitude contractions that convey fecal material to the rectum.
- The colon is highly active in transporting water and electrolytes, as well as products salvaged from undigested components of the meal by colonic bacteria.
- The colon maintains a life-long, mutually beneficial relationship with a vast bacterial ecosystem that metabolizes endogenous substances, nutrients, and drugs and protects the host from infection with pathogens.
- Defecation involves both involuntary and voluntary relaxation of muscle structures surrounding the anus and reflex pathways that control these structures.
|
 |
|