| The thyroid gland produces the prohormone tetraiodothyronine (T4) and the active hormone triiodothyronine (T3). Synthesis of T4 and T3 requires iodine, which can be a limiting factor in some parts of the world. Much of T3 is also made by peripheral conversion of T4 to T3, primarily through a nuclear receptor that regulates gene expression. T3 is critical for normal brain development and has broad effects on metabolism and cardiovascular function in adults.
|
| ANATOMY AND HISTOLOGY OF THE THYROID GLAND
|
| The thyroid gland is composed of a right lobe and a left lobe that sit anterolateral to the trachea (Fig. 41-1). Normally, the lobes of the thyroid gland are connected by a midventral isthmus. The thyroid gland receives a rich blood supply. It is drained by three sets of veins on each side: the superior, middle, and inferior thyroid veins. The thyroid gland receives sympathetic innervation that is vasomotor but not secretomotor.
|
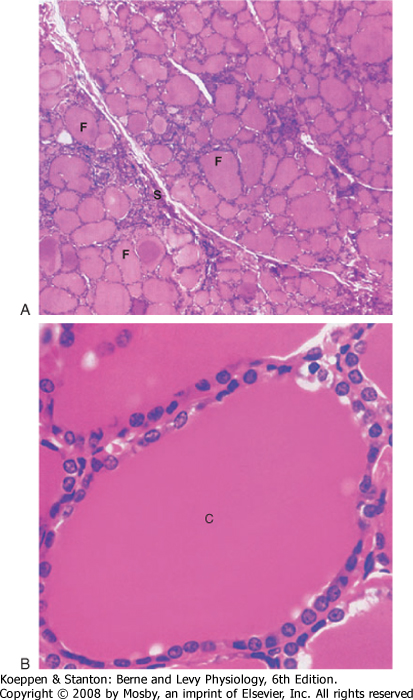
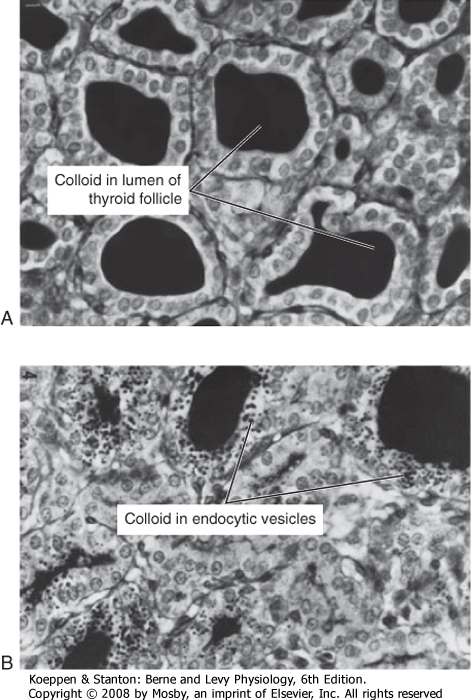
| The functional unit of the thyroid gland is the thyroid follicle, a spherical structure about 200 to 300 μm in diameter that is surrounded by a single layer of thyroid epithelial cells (Fig. 41-2). The epithelium sits on a basal lamina, the outermost structure of the follicle, and is surrounded by a rich capillary supply. The apical side of the follicular epithelium faces the lumen of the follicle. The follicular lumen itself is filled with colloid, which is composed of thyroglobulin; thyroglobulin is secreted and iodinated by the thyroid epithelial cells. The size of the epithelial cells and the amount of colloid are dynamic features that change with activity of the gland. The thyroid gland contains another type of cell in addition to follicular cells. Scattered within the gland are parafollicular cells called C cells. These cells are the source of the polypeptide hormone calcitonin, which is discussed in Chapter 39.
|
| PRODUCTION OF THYROID HORMONES
|
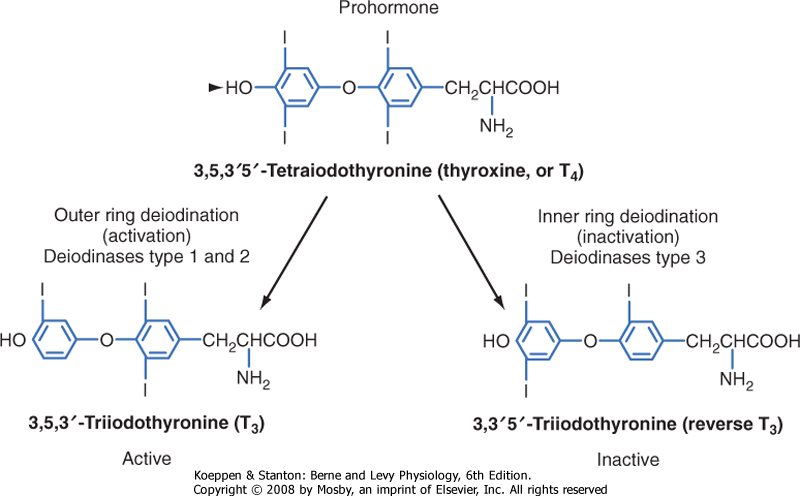
| The secretory products of the thyroid gland are iodothyronines (Fig. 41-3), a class of hormones formed by the coupling of two iodinated tyrosine molecules. Approximately 90% of the thyroid's output is 3,5,3',5'-tetraiodothyronine (thyroxine, or T4). T4 is primarily a prohormone. About 10% is 3,5,3'-triiodothyronine (T3), which is the active form of thyroid hormone. Less than 1% of thyroid output is 3,3',5'-triiodothyronine (reverse T3, or rT3), which is inactive. Normally, these three hormones are secreted in the same proportions as they are stored in the gland.
|
| Because the primary product of the thyroid gland is T4, yet the active form of thyroid hormone is T3, the thyroid axis relies heavily on peripheral conversion through the action of thyronine-specific deiodinases (Fig. 41-3). Most conversion of T4 to T3 by type 1 deiodinase occurs in tissues with high blood flow and rapid exchanges with plasma, such as the liver, kidneys, and skeletal muscle. This process supplies circulating T3 for uptake by other tissues in which local T3 generation is too low to provide sufficient thyroid hormone. Type 1 deiodinase is also expressed in the thyroid (again, where T4 is abundant) and has relatively low affinity (i.e., a Km of 1 μM) for T4. Levels of type 1 deiodinase are paradoxically increased in hyperthyroidism and contribute to the elevated circulating T3 levels in this disease.
|
| The brain maintains constant intracellular levels of T3 by a high-affinity deiodinase called type 2 deiodinase that is expressed in glial cells in the central nervous system. Type 2 deiodinase has a Km of 1 nM and maintains intracellular concentrations of T3 even when free T4 falls to low levels. Type 2 deiodinase is also present in the thyrotropes of the pituitary. In the pituitary, type 2 deiodinase acts as a "thyroid axis sensor" that mediates the ability of circulating T4 to feed back on secretion of thyroid-stimulating hormone (TSH) (see later). Expression of type 2 deiodinase is increased during hypothyroidism, which helps maintain constant T3 levels in the brain.
|
| There is also an "inactivating" deiodinase called type 3 deiodinase. Type 3 deiodinase is a high-affinity, inner ring deiodinase that converts T4 to the inactive rT3. Type 3 deiodinase is increased during hyperthyroidism, which helps blunt the overproduction of T4. All forms of iodothyronines are eventually further deiodinated to noniodinated thyronine.
|
| page 725 |  | | page 726 |
| Figure 41-1 A and B, Anatomy of the thyroid gland. C, Image of pertechnetate uptake by a normal thyroid gland. (Modified from Drake RL et al: Gray's Anatomy for Students. Philadelphia, Churchill Livingstone, 2005.) |
| Because of the unique role of iodide in thyroid physiology, a description of thyroid hormone synthesis requires some understanding of iodide turnover
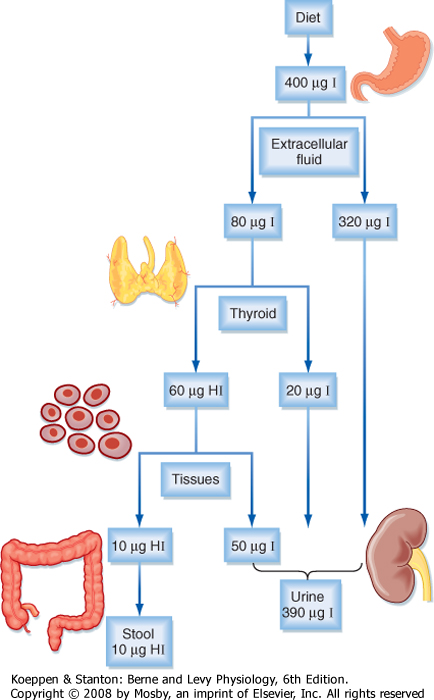
(Fig. 41-4). An average of 400 μg of iodide per person is ingested daily in the United States versus a minimum daily requirement of 150 μg for adults, 90 to 120 μg for children, and 200 μg for pregnant women. In the steady state, virtually the same amount, 400 μg, is excreted in urine. Iodide is actively concentrated in the thyroid gland, salivary glands, gastric glands, lacrimal glands, mammary glands, and choroid plexus. About 70 to 80 μg of iodide is taken up daily by the thyroid gland from a circulating pool that contains approximately 250 to 750 μg of iodide. The total iodide content of the thyroid gland averages 7500 μg, virtually all of which is in the form of iodothyronines. In the steady state, 70 to 80 μg of iodide, or about 1% of the total, is released from the gland daily. Of this amount, 75% is secreted as thyroid hormone and the remainder as free iodide. The large ratio (100 : 1) of iodide stored in the form of hormone to the amount turned over daily protects the individual from the effects of iodide deficiency for about 2 months. Iodide is also conserved by a marked reduction in the renal excretion of iodide as its concentration in serum falls.
|
| Overview of Thyroid Hormone Synthesis
|
| page 726 |  | | page 727 |
| Figure 41-2 Histology of the thyroid gland at low (upper panel) and high (lower panel) magnification. C, colloid; F, thyroid follicles; S, connective tissue septa. (From Young B et al: Wheater's Functional Histology, 5th ed. Philadelphia, Churchill Livingstone, 2006.) |
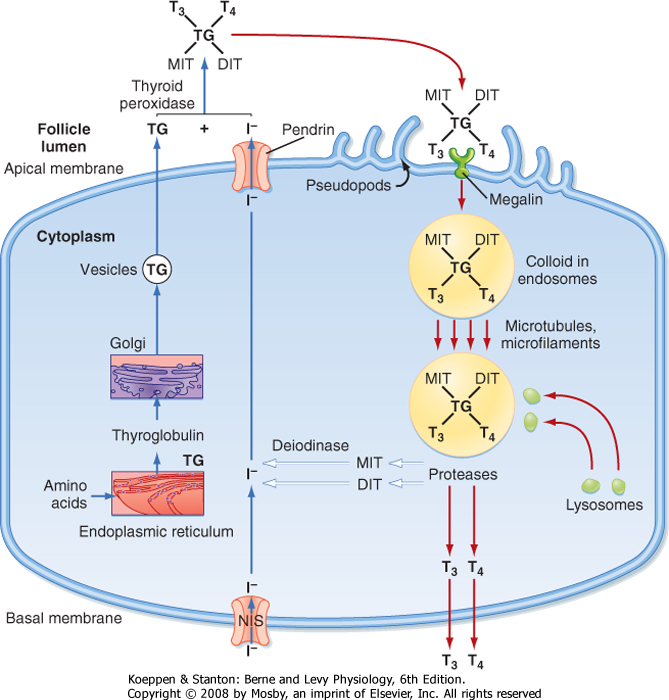
| To understand thyroid hormone synthesis and secretion, one must appreciate the directionality of each process as it relates to the polarized thyroid epithelial cell (Fig. 41-5). Synthesis of thyroid hormone requires two precursors: iodide and thyroglobulin. Iodide is transported across cells from the basal (vascular) side to the apical (follicular luminal) side of the thyroid epithelium. Amino acids are assembled by translation into thyroglobulin, which is then secreted from the apical membrane into the follicular lumen. Thus, synthesis involves a basal-to-apical movement of precursors into the follicular lumen (Fig. 41-5, blue arrows). Actual synthesis of iodothyronines occurs enzymatically in the follicular lumen close to the apical
membrane of the epithelial cells (see later). Secretion involves receptor-mediated endocytosis of iodinated thyroglobulin and apical-to-basal movement of the endocytic vesicles and their fusion with lysosomes. Thyroglobulin is then enzymatically degraded, which results in the release of thyroid hormones from the thyroglobulin peptide backbone. Finally, thyroid hormones move across the basolateral membrane, probably through a specific transporter, and ultimately into blood. Thus, secretion involves apical-to-basal movement (Fig. 41-5, red arrows). There are also scavenger pathways within the epithelial cell that reuse iodine and amino acids after enzymatic digestion of thyroglobulin (Fig. 41-5, white arrows).
|
| Synthesis of Iodothyronines within a Thyroglobulin Backbone
|
| Iodide is actively transported into the gland against chemical and electrical gradients by a 2Na+-1I- symporter) (NIS) located in the basolateral membrane of thyroid epithelial cells. Normally, a thyroid-plasma free iodide ratio of 30 is maintained. This so-called iodide trap requires the generation of energy by oxidative phosphorylation and displays saturation kinetics. NIS is highly expressed in the thyroid gland, but it is also expressed at lower levels in the placenta, salivary glands, and actively lactating breast. One iodide ion is transported uphill against an iodide gradient while two sodium ions move down the electrochemical gradient from extracellular fluid into the thyroid cell. The energy source for this secondary active transporter is provided by Na+,K+-ATPase in the plasma membrane. Expression of the NIS gene is inhibited by iodide and stimulated by TSH. Numerous inflammatory cytokines also suppress NIS gene expression. A reduction in dietary iodide intake depletes the circulating iodide pool and greatly enhances the activity of the iodide trap. When dietary iodide intake is low, the percentage of thyroid uptake of iodide can reach 80% to 90%.
|
| The steps in thyroid hormone synthesis are shown in Figure 41-6. After entering the gland, iodide rapidly moves to the apical plasma membrane of epithelial cells. From there, iodide is transported into the lumen of the follicles by a sodium-independent iodide/chloride transporter named pendrin. Iodide is immediately oxidized to iodine and incorporated into tyrosine molecules (Fig. 41-5). The iodinated tyrosine molecules are not free in solution (Fig. 41-6) but are incorporated by peptide linkages within the protein thyroglobulin. Thyroglobulin is continually exocytosed into the follicular lumen and is iodinated to form both monoiodotyrosine (MIT) and diiodotyrosine (DIT) (see Fig. 41-6). After iodination, two DIT molecules are coupled to form T4, or one MIT molecule and one DIT molecule are coupled to form T3. Coupling also occurs between iodinated tyrosines that remain part of the primary structure of thyroglobulin. This entire sequence of reactions is catalyzed by thyroid peroxidase (TPO), an enzyme complex that spans the apical membrane. The immediate oxidant (electron acceptor) for the reaction is hydrogen peroxide (H2O2). The mechanism whereby H2O2 is generated in the thyroid gland involves NADPH oxidase, which is also localized to the apical membrane.
|
| When the availability of iodide is restricted, the formation of T3 is favored. Because T3 is three times as potent as T4, this response provides more active hormone per molecule of organified iodide. The proportion of T3 is also increased when the gland is hyperstimulated by TSH or other activators.
|
| Secretion of Thyroid Hormones
|
| page 727 |  | | page 728 |
| Figure 41-3 Structure of the iodothyronines T4, T3, and reverse T3. |
| Figure 41-4 Iodine distribution and turnover in humans. HI = hormone-associated iodine. |
| Several transporters mediate transport of thyroid hormones across cell membranes Thyroid hormone transporters include sodium/taurocholate-cotransporting polypeptides (NCTPs), organic anion-transporting polypeptides (OATPs), L-type amino acid transporters (LATs), and the monocarboxylate transporters (MCTs). These transporters show specificity with respect to T4 versus T3 binding and cell-specific expression. Recently, mutations in MCT8 have been linked to human disease that may be due to an intracellular deficit of thyroid hormone, elevated T3 levels, and severe psychomotor retardation. |
| Once thyroglobulin has been iodinated, it is stored in the lumen of the follicle as colloid (Fig. 41-2). Release of T4 and T3 into the bloodstream requires binding of thyroglobulin to the receptor megalin, followed by endocytosis and lysosomal degradation of thyroglobulin
(Fig. 41-7; also see Fig. 41-5). Enzymatically released T4 and T3 then leave the basal side of the cell and enter the blood.
|
| The MIT and DIT molecules, which also are released during proteolysis of thyroglobulin, are rapidly deiodinated within the follicular cell by the enzyme intrathyroidal deiodinase (Fig. 41-5; white arrows). This deiodinase is specific for MIT and DIT and cannot utilize T4 and T3 as substrates. The iodide is then recycled into synthesis of T4 and T3. Amino acids from the digestion of thyroglobulin reenter the intrathyroidal amino acid pool and can be reused for protein synthesis (Fig. 41-5, white arrows). Only minor amounts of intact thyroglobulin leave the follicular cell under normal circumstances.
|
| TRANSPORT AND METABOLISM OF THYROID HORMONES
|
| page 728 |  | | page 729 |
| Figure 41-5 Synthesis (blue arrows) and secretion (red arrows) of thyroid hormones by the thyroid epithelial cell. White arrows denote pathways involved in the conservation of iodine and amino acids. |
| Figure 41-6 Reactions involved in the generation of iodide, MIT, DIT, T3, and T4. |
| page 729 |  | | page 730 |
| Figure 41-7 Before (A) and minutes after (B) rapid induction of thyroglobulin endocytosis by TSH. (From Wollman SH et al: J Cell Biol 21:191, 1964.) |
|
Table 41-1.
Average Thyroid Hormone Turnover |
| | T4 | T3 | rT3 |
| Daily production (μg) | 90 | 35 | 35 |
| From thyroid (%) | 100 | 25 | 5 |
| From T4 (%) | - | 75 | 95 |
| Extracellular pool (μg) | 850 | 40 | 40 |
| Plasma concentration | | | |
| Total (μg/dL) | 8.0 | 0.12 | 0.04 |
| Free (ng/dL) | 2.0 | 0.28 | 0.20 |
| Half-life (days) | 7 | 1 | 0.8 |
| Metabolic clearance (L/day) | 1 | 26 | 77 |
| Fractional turnover per day (%) | 10 | 75 | 90 |
| Secreted T4 and T3 circulate in the bloodstream almost entirely bound to proteins. Normally, only about 0.03% of total plasma T4 and 0.3% of total plasma T3 exist in
the free state (Table 41-1). Free T3 is biologically active and mediates the effects of thyroid hormone on peripheral tissues, in addition to exerting negative feedback on the pituitary and hypothalamus (see later). The major binding protein is thyroxine-binding globulin (TBG). TBG is synthesized in the liver and binds one molecule of T4 or T3.
|
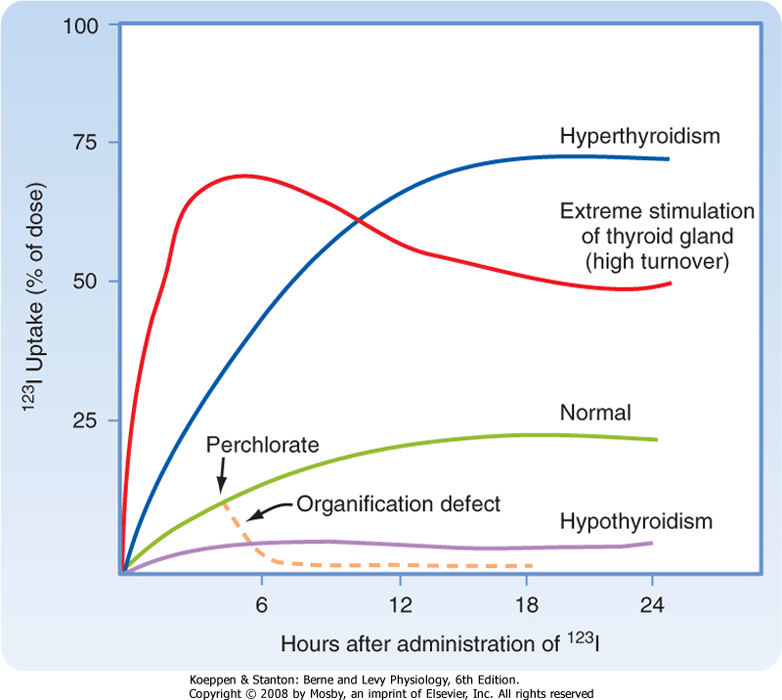
| Because of its ability to trap and incorporate iodine into thyroglobulin (called organification), the activity of the thyroid can be assessed by radioactive iodine uptake (RAIU). In this test a tracer dose of 123I is administered and RAIU is measured by placing a gamma detector on the neck at 4 to 6 hours and at 24 hours. In the United States, where the diet is relatively rich in iodine, RAIU is about 15% after 6 hours and 25% after 24 hours (Fig. 41-8). Abnormally high RAIU (>60%) after 24 hours indicates hyperthyroidism. Abnormally low RAIU (<5%) after 24 hours indicates hypothyroidism. In individuals with extreme chronic stimulation of the thyroid (Graves' disease-associated thyrotoxicosis), iodide is trapped, organified, and released as hormone very rapidly. In these cases of elevated turnover, 6-hour RAIU will be very high, but 24-hour RAIU will be lower (Fig. 41-8). A number of anions, such as thiocyanate (CNS-), perchlorate (HClO4-), and pertechnetate (TcO4-), are competitive or noncompetitive inhibitors of iodide transport via NIS. If iodide cannot be rapidly incorporated into tyrosine (organification defect) after its uptake by the cell, administration of one of these anions will, by blocking further iodide uptake, cause rapid release of iodide from the gland (Fig. 41-8). This release occurs as a result of the high thyroidplasma concentration gradient. |
| The thyroid can be imaged with a rectilinear scanner or gamma camera after the administration of a tracer, 123I, 131I, or the iodine-mimic pertechnetate (99mTc). Imaging can display the size and shape of the thyroid (Fig. 41-1, C), as well as heterogeneities of active versus inactive tissue within the thyroid gland. Such heterogeneities are often due to the development of thyroid nodules, which are regions of enlarged follicles with evidence of regressive changes-indicative of cycles of stimulation and involution. Particular "hot" nodules (i.e., nodules that display high RAIU on imaging) are not usually cancerous but may lead to thyrotoxicosis (hyperthyroidism-see later). "Cold" nodules are 10 times more likely to be cancerous than "hot" nodules. Such nodules can be sampled for pathological analysis by fine-needle aspiration biopsy. |
| The thyroid can also be imaged by ultrasonography, which is superior in resolution to RAIU imaging. Ultrasonography is used to guide the physician during fine-needle aspiration biopsy of a nodule. The highest resolution of the thyroid is achieved with magnetic resonance imaging (MRI). |
| page 730 |  | | page 731 |
| Figure 41-8 Thyroid gland iodothyronine uptake curves for normal, hypothyroid, hyperthyroid, and defective organification states. |
| About 70% of circulating T4 and T3 is bound to TBG; 10% to 15% is bound to another specific thyroid-binding protein called transthyretin (TTR). Albumin binds 15% to 20%, and 3% is bound to lipoproteins. Ordinarily, only alterations in TBG concentration significantly affect total plasma T4 and T3 levels. Two important biological functions have been ascribed to
TBG. First, it maintains a large circulating reservoir of T4 that buffers any acute changes in thyroid gland function. Second, binding of plasma T4 and T3 to proteins prevents the loss of these relatively small hormone molecules in urine and thereby helps conserve iodide. TTR, in particular, provides thyroid hormones to the central nervous system.
|
| PHYSIOLOGICAL EFFECTS OF THYROID HORMONE
|
| Thyroid hormone acts on essentially all cells and tissues, and imbalances in thyroid function constitute some of the most common endocrine diseases. Thyroid hormone has many direct actions, but it also acts in more subtle ways to optimize the actions of several other hormones and neurotransmitters.
|
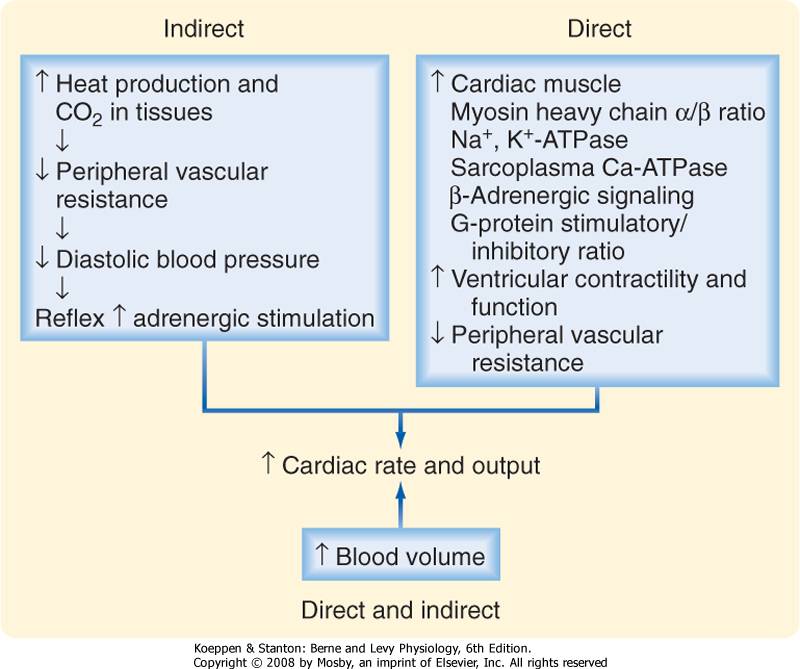
| Figure 41-9 Mechanisms by which thyroid hormone increases cardiac output. The indirect mechanisms are probably quantitatively more important. |
| Perhaps the most clinically important actions of thyroid hormone are those on cardiovascular physiology. T3 increases cardiac output, thereby ensuring sufficient delivery of O2 to tissues (Fig. 41-9). The resting heart rate and stroke volume are increased. The speed and force of myocardial contractions are enhanced (positive chronotropic and inotropic effects, respectively), and the diastolic relaxation time is shortened (positive lusitropic effect). Systolic blood pressure is modestly augmented and diastolic blood pressure is decreased. The resultant widened pulse pressure reflects the combined effects of the increased stroke volume and the reduction in total peripheral vascular resistance secondary to blood vessel dilation in skin, muscle, and heart. These effects, in turn, are partly due to the increase in tissue production of heat and CO2 that thyroid hormone induces (see later). In addition, however, thyroid hormone decreases systemic
vascular resistance by dilating resistance arterioles in the peripheral circulation. Total blood volume is increased by activating the renin-angiotensin-aldosterone axis and thereby increasing renal tubular sodium reabsorption (see Chapter 33).
|
| page 731 |  | | page 732 |
| Thyroid hormone levels in the normal range are necessary for optimum cardiac performance. A deficiency of thyroid hormone in humans reduces stroke volume, left ventricular ejection fraction, cardiac output, and the efficiency of cardiac function. The latter defect is shown by the fact that the stroke work index [(stroke volume/left ventricular mass) × peak systolic blood pressure] is decreased even more than myocardial oxidative metabolism is. The rise in systemic vascular resistance may contribute to this cardiac debility. In contrast, excess thyroid hormone enhances cardiac output and increases the uncoupling proteins UCP-2 and UCP-3 in cardiac muscle; these proteins uncouple ATP production from O2 utilization during the β oxidation of free fatty acids. This can cause high-output cardiac failure. When hyperthyroidism develops in aging individuals, the cardiac effects of thyroid hormone may include rapid atrial arrhythmias, flutter, and fibrillation (see Chapter 15). |
 |
| The cardiac inotropic effects of T3 are indirect, via enhanced responsiveness to catecholamines (see Chapter 42), and direct (Fig. 41-9). Myocardial calcium uptake is increased, which enhances contractile force. Thyroid hormone inhibits expression of the Na+-Ca++ antiporter, thereby increasing intramyocellular [Ca++]. T3 increases the velocity and strength of myocardial contraction. It also increases ryanodine Ca++ channels
in the sarcoplasmic reticulum, which promotes release of Ca++ from the sarcoplasmic reticulum during systole. Sarcoplasmic reticulum Ca++-ATPase (SERCA) is increased by T3, and as a result, sequestration of calcium during diastole is facilitated and the relaxation time is shortened.
|
| Effects on Basal Metabolic Rate
|
| Thyroid hormones increase the basal rate of oxygen consumption and heat production (e.g., basal metabolic rate). As mentioned earlier, thyroid hormone increases the expression of mitochondrial uncoupling proteins (UCPs). This action is demonstrated in all tissues except the brain, gonads, and spleen. Glucose and fatty acid uptake and oxidation are increased overall, as are lactate-glucose and fatty acid-triglyceride recycling. Thyroid hormone does not augment diet-induced O2 utilization, and it may not change the efficiency of energy use during exercise.
|
| Thermogenesis must also increase concomitantly with O2 use. Thus, changes in body temperature parallel fluctuations in availability of thyroid hormone. The potential increase in body temperature, however, is moderated by a compensatory increase in heat loss through appropriate thyroid hormone-mediated increases in blood flow, sweating, and ventilation. Hyperthyroidism is accompanied by heat intolerance, whereas hypothyroidism is accompanied by cold intolerance.
|
| Increased O2 use ultimately depends on an increased supply of substrates for oxidation. T3 augments glucose absorption from the gastrointestinal tract and increases glucose turnover (glucose uptake, oxidation, and synthesis). In adipose tissue, thyroid hormone induces enzymes for the synthesis of fatty acids, acetyl-CoA carboxylase, and fatty acid synthase and enhances lipolysis by increasing the number of β-adrenergic receptors (see later). Thyroid hormone also enhances the clearance of chylomicrons. Thus, lipid turnover (FFA release from adipose tissue and oxidation) is augmented.
|
| Protein turnover (release of muscle amino acids, protein degradation and, to a lesser extent, protein synthesis and urea formation) is also increased. T3 potentiates the respective stimulatory effects of epinephrine, norepinephrine, glucagon, cortisol, and growth hormone on gluconeogenesis, lipolysis, ketogenesis, and proteolysis of the labile protein pool. The overall metabolic effect of thyroid hormone has been aptly described as accelerating the response to starvation. In addition, thyroid hormone stimulates the synthesis of cholesterol, but more so its oxidation and biliary secretion. The net effect is a decrease in the body pool and plasma levels of total and low-density lipoprotein cholesterol.
|
| The metabolic clearance of adrenal and gonadal steroid hormones, some B vitamins, and certain administered drugs is also increased by thyroid hormone.
|
| Thyroid hormone stimulates O2 utilization and also enhances O2 supply. Appropriately, T3 increases the resting respiratory rate, minute ventilation, and the ventilatory response to hypercapnia and hypoxia. These actions maintain a normal arterial Po2 when O2 utilization is increased and a normal Pco2 when CO2 production is increased. Additionally, the hematocrit increases slightly and thereby enhances the O2-carrying capacity. This increase in red blood cell mass results from stimulation of erythropoietin production by the kidney.
|
| Normal function of skeletal muscles also requires optimal amounts of thyroid hormone. This requirement may be related to the regulation of energy production and storage. Glycolysis and glycogenolysis are increased and glycogen and creatine phosphate are reduced by an excess of T4 and T3. The inability of muscle to take up and phosphorylate creatine leads to increased urinary excretion of creatine.
|
| Effects on the Autonomic Nervous System and Catecholamine Action
|
| There is synergism between catecholamines and thyroid hormones. Thyroid hormones are synergistic with catecholamines in increasing the metabolic rate, heat production, heart rate, motor activity, and excitation of the central nervous system. T3 may enhance sympathetic nervous system activity by increasing the number of β-adrenergic receptors in heart muscle and the generation of intracellular second messengers, such as cAMP.
|
| Effects on Growth and Maturation
|
| page 732 |  | | page 733 |
| Another major effect of thyroid hormone is to promote growth and maturation. A small but crucial amount of thyroid hormone crosses the placenta, and the fetal thyroid axis becomes functional at midgestation. Thyroid hormone is extremely important for normal neurological development and proper bone formation in the fetus. In infants, insufficient fetal thyroid
hormone causes cretinism, characterized by irreversible mental retardation and short stature (see later).
|
| Effects on Bone, Hard Tissue, and Dermis
|
| Thyroid hormone stimulates endochondral ossification, linear growth of bone, and maturation of the epiphyseal bone centers. T3 enhances the maturation and activity of chondrocytes in the cartilage growth plate, in part by increasing local growth factor production and action. Although thyroid hormone is not required for linear growth until after birth, it is essential for normal maturation of growth centers in the bones of the developing fetus. T3 also stimulates adult bone remodeling.
|
| The progression of tooth development and eruption depends on thyroid hormone, as does the normal cycle of growth and maturation of the epidermis, its hair follicles, and nails. The normal degradative processes in these structural and integumentary tissues are also stimulated by thyroid hormone. Thus, either too much or too little thyroid hormone can lead to hair loss and abnormal nail formation.
|
| Thyroid hormone alters the structure of subcutaneous tissue by inhibiting the synthesis and increasing the degradation of mucopolysaccharides (glycosaminoglycans) and fibronectin in the extracellular connective tissue.
|
| Effects on the Nervous System
|
| Thyroid hormone regulates the timing and pace of development of the central nervous system. Thyroid hormone deficiency in utero and in early infancy decreases growth of the cerebral and cerebellar cortex, proliferation of axons and branching of dendrites, synaptogenesis, myelinization, and cell migration. Irreversible brain damage results when thyroid hormone deficiency is not recognized and treated promptly after birth. The structural defects just described are paralleled by biochemical abnormalities. Decreased thyroid hormone levels reduce cell size, RNA and protein content, tubulin- and microtubule-associated protein, protein and lipid content of myelin, local production of critical growth factors, and rates of protein synthesis.
|
| Thyroid hormone also enhances wakefulness, alertness, responsiveness to various stimuli, auditory sense, awareness of hunger, memory, and learning capacity. In addition, normal emotional tone depends on proper thyroid hormone availability. Furthermore, the speed and amplitude of peripheral nerve reflexes are increased by thyroid hormone, as is motility of the gastrointestinal tract.
|
| Effects on Reproductive Organs and Endocrine Glands
|
| Hypothyroidism refers to insufficient production of thyroid hormones and can occur as primary, secondary, or tertiary endocrine disease (see Chapter 40). In primary hypothyroidism, T4 and T3 levels are abnormally low, and TSH is high (see later). In secondary and tertiary hypothyroidism, both thyroid hormones and TSH are low. The response of TSH levels to synthetic TRH can be used to distinguish between pituitary and hypothalamic disease. |
| Hypothyroidism in the fetus or early childhood leads to cretinism. Affected individuals have severe mental retardation, short stature with incomplete skeletal development, coarse facial features, and a protruding tongue. The most common cause of hypothyroidism in children is iodide deficiency. Iodide is not plentiful in the environment, and deficiency of iodide is a major cause of hypothyroidism in certain mountainous regions of South America, Africa, and Asia. This tragic form of endemic cretinism can easily be prevented by public health programs that add iodide to table salt or that provide yearly injections of a slowly absorbed iodide preparation. Congenital defects are a less common cause of neonatal/child hypothyroidism. In most cases, the thyroid gland simply does not develop (thyroid gland dysgenesis). Less frequent causes of childhood hypothyroidism are mutations in genes involved in thyroid hormone production (e.g., genes for NIS, TPO, thyroglobulin, and pendrin) and blocking antibodies to the TSH receptor. The severity of the neurological and skeletal defects is closely linked to the time of diagnosis and thyroid hormone (T4) replacement treatment, with early treatment resulting in a normal IQ and subtle neurological deficits. Hypothyroid babies usually appear normal at birth because of maternal thyroid hormones. However, in geographical areas of endemic iodide deficiency, even the mother may be somewhat hypothyroid and unable to make up for the fetal defects. Alternatively, maternal hypothyroidism can cause mild mental retardation in euthyroid fetuses. Neonatal screening (T4 or TSH levels) has played a major role in the prevention of severe cretinism. If hypothyroidism at birth remains untreated for only 2 to 4 weeks, the central nervous system will not mature normally in the first year of life. Developmental milestones such as sitting, standing, and walking will be late, and severe irreversible mental retardation can result. |
| Hypothyroidism in adults who are not iodide deficient most often results from idiopathic atrophy of the gland, which is thought to be preceded by a chronic autoimmune inflammatory reaction. In this form of lymphocytic thyroiditis (Hashimoto's disease), the antibodies that are produced may block hormone synthesis or growth of the thyroid gland, or they may have cytotoxic properties. Other causes of hypothyroidism include iatrogenic causes (e.g., radiochemical damage or surgical removal for treatment of hyperthyroidism), nodular goiters, and pituitary or hypothalamic disease. |
| page 733 |  | | page 734 |
| The clinical picture of hypothyroidism in adults is in many respects the exact opposite of that seen in hyperthyroidism. The lower than normal metabolic rate leads to weight gain without an appreciable increase in caloric intake. The decreased thermogenesis lowers body temperature and causes intolerance to cold, decreased sweating, and dry skin. Adrenergic activity is decreased, and therefore bradycardia may occur. Movement, speech, and thought are all slowed, and lethargy, sleepiness, and lowering of the upper eyelids (ptosis) occur. An accumulation of mucopolysaccharides-extracellular matrix-in tissues also causes an accumulation of fluid. This nonpitting myxedema produces puffy features; an enlarged tongue; hoarseness; joint stiffness; effusions in the pleural, pericardial, and peritoneal spaces; and pressure on peripheral and cranial nerves, entrapped by excess ground substance, with consequent thyroid dysfunction. Constipation, loss of hair, menstrual dysfunction, and anemia are other signs. In adults lacking thyroid hormone, positron emission tomography demonstrates a generalized reduction in cerebral blood flow and glucose metabolism. This abnormality may explain the psychomotor retardation and depressed affect of hypothyroid individuals. |
| Replacement therapy with T4 is curative in adults. T3 is not needed because it will be generated intracellularly from the administered T4. Furthermore, giving T3 raises plasma T3 to nonphysiological levels. |
| In both women and men, thyroid hormone plays an important, permissive role in the regulation of reproductive function. The normal ovarian cycle of follicular development, maturation, and ovulation, the homologous testicular process of spermatogenesis, and maintenance of the healthy pregnant state are all disrupted by significant deviations in thyroid hormone levels from the normal range. In part, these deleterious effects may be caused by alterations in the metabolism
or availability of steroid hormones. For example, thyroid hormone stimulates hepatic synthesis and release of sex steroid-binding globulin.
|
| Thyroid hormone also has significant effects on other parts of the endocrine system. Pituitary production of growth hormone is increased by thyroid hormone, whereas that of prolactin is decreased. Adrenocortical secretion of cortisol (see Chapter 42), as well as metabolic clearance of this hormone, is stimulated, but plasma free cortisol levels remain normal. The ratio of estrogens to androgens (see Chapter 43) is increased in men (in whom breast enlargement may occur with hyperthyroidism). Decreases in both parathyroid hormone and 1,25-(OH)2-vitamin D production are compensatory consequences of the effects of thyroid hormone on bone resorption (see Chapter 39).
|
| Kidney size, renal plasma flow, glomerular filtration rate, and transport rates for a number of substances are also increased by thyroid hormone.
|
| Mechanism of Thyroid Hormone Action
|
|
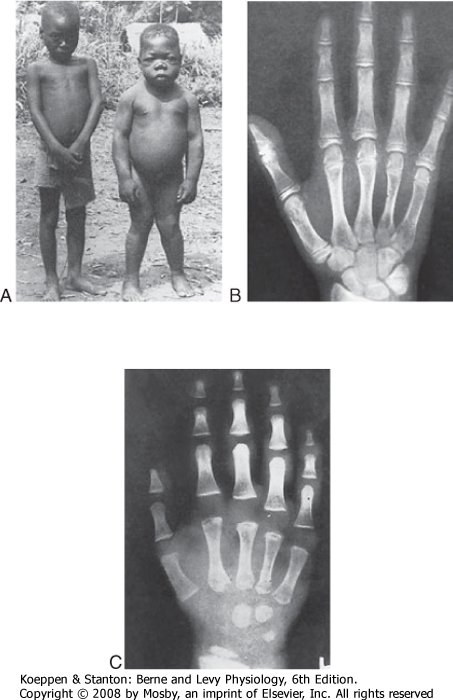
| Figure 41-10 A, Normal 6-year-old child (left) and a congenitally hypothyroid 17-year-old child (right) from the same village in an area of endemic cretinism. Note especially the short stature, obesity, malformed legs, and dull expression of the mentally retarded hypothyroid child. Other features are a prominent abdomen, a flat broad nose, a hypoplastic mandible, dry scaly skin, delayed puberty, and muscle weakness. (From Delange FM. In Braverman LE, Utiger RD [eds]: Werner and Ingbar's the Thyroid, 7th ed. Philadelphia, Lippincott-Raven, 1996.) X-ray films of the hand of a normal 13-year-old child (B) and that of a 13-year-old child suffering from hypothyroidism (C). Note that the child with hypothyroidism has a marked delay in development of the small bones of the hands, in growth centers at either end of the fingers, and in the growth center of the distal end of the radius. (B, From Tanner JM et al: Assessment of Skeletal Maturity and Prediction of Adult Height (TW2 Method). New York, Academic Press, 1975; C, from Andersen HJ. In Gardner LI [ed]: Endocrine and Genetic Diseases of Childhood and Adolescence. Philadelphia, Saunders, 1975.) |
| Free T4 and T3 enter cells by a carrier-mediated, energy-dependent process. Transport of T4 is rate limiting for the intracellular production of T3. Within the cell, most, if not all of the T4 is converted to T3 (or rT3). Many, but not all T3 actions are mediated through its binding to one of the members of the thyroid hormone receptor (TR) family (Fig. 41-10, A). The TR family
belong to the nuclear hormone receptor superfamily of transcription factors (see also Chapters 3 and 39).
|
| REGULATION OF THYROID FUNCTION
|
| page 734 |  | | page 735 |
| In humans there are two TR genes, THRA and THRB, located on chromosomes 17 and 3, respectively, that encode the classic nuclear thyroid hormone receptors. THRA encodes TRα, which is alternatively spliced to form two main isoforms. TRα-1 is a bona fide TR, whereas the other isoform does not bind T3. THRB encodes TRβ-1 and TRβ-2, both of which are high-affinity receptors for T3. The tissue distribution of TRα-1 and TRβ-1 is widespread. TRα-1 is especially expressed in cardiac and skeletal muscle, and TRα-1 is the dominant TR that transduces thyroid hormone action on the heart. By contrast, TRβ-1 is expressed more in the brain, liver, and kidney. TRβ-2 expression is restricted to the pituitary and critical areas of the hypothalamus, as well as the cochlea and retina. T3-bound TRβ-2 is responsible for inhibiting expression of the prepro-TRH gene in the paraventricular neurons of the hypothalamus and the β subunit TSH gene in pituitary thyrotropes. Thus, the negative-feedback effects of thyroid hormone on both TRH and TSH secretion are largely mediated by TRβ-2. T3 also down-regulates TRβ-2 gene expression in the pituitary gland. |
| The unliganded form of the TR-RXR dimer interacts with several corepressor proteins, including NCoR, SMRT, and Alien. On hormone binding, corepressors are released, and coactivators are recruited to the hormone-receptor complex. The two major coactivator proteins are the SRC family (SRC-1, SRC-2, and SRC-3) and the DRIP-TRAP complex. |
| An understanding of TR subtypes and tissue expression is of more than academic interest because inactivating mutant genes have increasingly been found to be causes of clinical syndromes manifested by resistance to thyroid hormone (RTH syndrome). The most common mutations occur in the TRβ-2 subtype. In these patients there is incomplete negative thyroid hormone feedback at the hypothalamic-pituitary level. Thus, T4 levels are elevated, but TSH is not suppressed. When the resistance is purely at the hypothalamic-pituitary level, the patient may exhibit signs of hyperthyroidism because of the excess effects of high thyroid hormone levels on peripheral tissue, particularly on the heart through TRα-1. These individuals have clinical signs such as goiter, short stature, decreased weight, tachycardia, hearing loss, monochromatic vision, and decreased IQ. |
 |
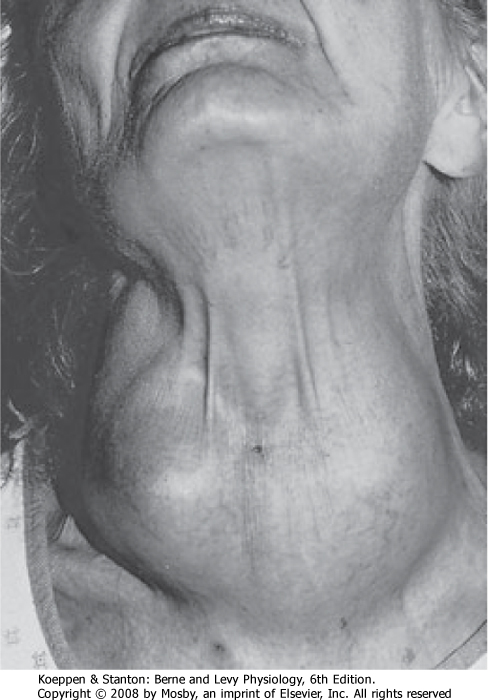
|
| Figure 41-11 The thyroid gland is located in the anterior aspect of the neck, where it is easily visualized and palpated when enlarged (goiter). |
| The most important regulator of thyroid gland function and growth is the hypothalamic-pituitary thyroid-releasing hormone-thyroid-stimulating hormone
axis (see Chapter 40, Fig. 40-13). TSH stimulates every aspect of thyroid function. TSH has immediate, intermediate, and long-term actions on the thyroid epithelium. Immediate actions of TSH include induction of pseudopod extension, endocytosis of colloid, and the formation of colloid droplets in the cytoplasm, which represent thyroglobulin within endocytic vesicles (see Fig. 41-7). Shortly thereafter, iodide uptake and TPO activity increase. Concurrently, TSH also stimulates
entry of glucose into the hexose monophosphate shunt pathway, which generates the NADPH that is needed for the peroxidase reaction. In addition, TSH stimulates the proteolysis of thyroglobulin and release of T4 and T3 from the gland. Intermediate effects of TSH on the thyroid gland occur after a delay of hours to days and involve protein synthesis and the expression of numerous genes, including those encoding NIS, thyroglobulin, TPO, and megalin. Sustained TSH stimulation leads to the long-term effects of hypertrophy and hyperplasia of follicular cells. Capillaries proliferate, and thyroid blood flow increases. These actions, which underlie the growth-promoting effects of TSH on the gland, are supported by the local production of growth factors. A noticeably enlarged thyroid gland is called a goiter (Fig. 41-11). One form of goiter is due to lack of adequate iodine in the diet, which results in low thyroid hormone and elevated TSH levels.
|
| page 735 |  | | page 736 |
| Graves' disease is the most common form of hyperthyroidism. It occurs most frequently between the ages of 20 and 50 and is 10 times more common in women than in men. Graves' disease is an autoimmune disorder in which autoantibodies are produced against the TSH receptor. The nature of the specific autoantibodies depends on the epitope that they are directed against. The most critical type is called the thyroid-stimulating immunoglobulin (TSI). The hyperthyroidism is often accompanied by a diffuse goiter as a result of hyperplasia and hypertrophy of the gland. The follicular epithelial cells become tall columnar cells, and the colloid shows a scalloped periphery indicative of rapid turnover. |
| The primary clinical state found in Graves' disease is thyrotoxicosis-the state of excessive thyroid hormone in blood and tissues. A patient with thyrotoxicosis presents one of the most striking pictures in clinical medicine. The large increase in metabolic rate is accompanied by the highly characteristic combination of weight loss despite increased intake of food. The increased heat production causes discomfort in warm environments, excessive sweating, and greater intake of water. The increase in adrenergic activity is manifested by a rapid heart rate, hyperkinesis, tremor, nervousness, and a wide-eyed stare. Weakness is caused by a loss of muscle mass, as well as by an impairment in muscle function. Other symptoms include a labile emotional state, breathlessness during exercise, and difficulty swallowing or breathing because of compression of the esophagus or trachea by the enlarged thyroid gland (goiter). The most common cardiovascular sign is sinus tachycardia. There is increased cardiac output associated with a widened pulse pressure secondary to a positive inotropic effect coupled with a decrease in vascular resistance. A major clinical sign in Graves' disease is exophthalmos (abnormal protrusion of the eyeball) and periorbital edema as a result of recognition by the anti-TSH receptor antibodies of a similar epitope within the orbital cells (probably fibroblasts). Graves' disease is diagnosed by an elevated serum free and total T4 or T3 level (i.e., thyrotoxicosis) and the clinical signs of diffuse goiter and ophthalmopathy. In most cases, uptake of iodine or pertechnetate by the thyroid is excessive and diffuse. Serum TSH levels are low because the hypothalamus and the pituitary gland are inhibited by the high levels of T4 and T3. Assay of TSH levels and for the presence of circulating TSI will distinguish Graves' disease (a primary endocrine disorder) from a rare adenoma of the pituitary thyrotrophs (a secondary endocrine disease). The latter condition generates elevated TSH levels, unaccompanied by TSI. |
| Treatment of Graves' disease is usually removal of the thyroid tissue, followed by lifelong replacement therapy with T4. Thyroid tissue can be ablated by either the radiation effects of 131I or surgery. Surgical removal of the gland rarely but potentially precipitates massive release of hormone that causes thyroid storm, which causes death in 30% of patients, primarily as a result of cardiac failure and arrhythmia. An alternative to removal of thyroid tissue is administration of antithyroid drugs that inhibit TPO activity. |
 |
| Regulation of thyroid hormone secretion by TSH is under exquisite negative-feedback control (see Chapter 40). Circulating thyroid hormones act on the pituitary gland to decrease TSH secretion, primarily by repressing TSH β subunit gene expression. The pituitary gland expresses the high-affinity type 2 deiodinase. Thus, small changes in free T4 in blood result in significant changes in intracellular T3 in the pituitary thyrotrope. Because the diurnal variation in TSH secretion is small, thyroid hormone secretion and plasma concentrations are relatively constant. Only small nocturnal increases in secretion of TSH and release of T4 occur. Thyroid hormones also feed back on hypothalamic thyroid-releasing hormone (TRH)-secreting neurons. In these neurons, T3 inhibits expression of the prepro-TRH gene. Another important regulator of thyroid gland function is iodide itself, which has a biphasic action. At relatively low levels of iodide intake, the rate of
thyroid hormone synthesis is directly related to the availability of iodide. However, if the intake of iodide exceeds 2 mg/day, the intraglandular concentration of iodide reaches a level that suppresses NADPH oxidase activity and the NIS and TPO genes and thereby the mechanism of hormone biosynthesis. This autoregulatory phenomenon is known as the Wolff-Chaikoff effect. As the intrathyroidal iodide level subsequently falls, NIS and TPO genes are derepressed and the production of thyroid hormone returns to normal. In unusual instances, the inhibition of hormone synthesis by iodide can be great enough to induce thyroid hormone deficiency. The temporary reduction in hormone synthesis by excess iodide can also be used therapeutically in hyperthyroidism.
|
| Thyroid hormones increase O2 utilization, energy expenditure, and heat production. Therefore, it is logical to expect that the availability of active thyroid hormone correlates with changes in the body's caloric and thermal status. In fact, ingestion of excess calories, particularly in the form of carbohydrate, increases the production and plasma concentration of T3, as well as the individual's metabolic rate, whereas prolonged fasting leads to corresponding decreases. Because most T3 arises from circulating T4 (Table 41-1), peripheral mechanisms are important in mediating these changes. However, starvation also gradually lowers T4 levels in humans.
|
| page 736 |  | | page 737 |
- The thyroid gland is situated in the ventral aspect of the neck and is composed of right and left lobes anterolateral to the trachea and connected by an isthmus.
- The thyroid gland is the source of tetraiodothyronine (thyroxine, T4) and triiodothyronine (T3).
- The basic endocrine unit in the gland is a follicle that consists of a single spherical layer of epithelial cells surrounding a central lumen that contains colloid or stored hormone.
- Iodide is taken up into thyroid cells by a sodium-iodide symporter in the basolateral plasma membrane.
- T4 and T3 are synthesized from tyrosine and iodide by the enzyme complex thyroid peroxidase. Tyrosine is incorporated in peptide linkages within the protein thyroglobulin. After iodination, two iodotyrosine molecules are coupled to yield the iodothyronines.
- Secretion of stored T4 and T3 requires retrieval of thyroglobulin from the follicle lumen by endocytosis. To support hormone synthesis, iodide is conserved by recycling the iodotyrosine molecules that escape coupling within thyroglobulin.
- More than 99.5% of T4 and T3 circulates bound to the following proteins: thyroid-binding globulin (TBG), transthyretin, and albumin. Only the free fractions of T4 and T3 are biologically active.
- T4 functions largely as a prohormone whose disposition is regulated by three types of deiodinases. Monodeiodination of the outer ring yields 75% of the daily production of T3, which is the principal active hormone. Alternatively, monodeiodination of the inner ring yields reverse T3, which is biologically inactive. Proportioning of T4 between T3 and reverse T3 regulates the availability of active thyroid hormone.
- T3 and, to a much lesser extent, T4 bind to thyroid hormone receptor (TR) subtypes linked to thyroid regulatory elements (TREs) in target DNA molecules. As a result, induction or repression of gene expression increases or decreases a large number of enzymes, as well as structural and functional proteins.
- Thyroid hormone increases and is a major regulator of the basal metabolic rate. Additional important actions of thyroid hormone are to increase the heart rate, cardiac output, and ventilation and to decrease peripheral resistance. The corresponding increase in heat production leads to increased sweating. Substrate mobilization and disposal of metabolic products are enhanced.
- Other thyroid hormone effects on the central nervous system and skeleton are crucial to normal growth and development. In the absence of the hormone, brain development is retarded and cretinism results. The stature shortens and the bones fail to mature. In adults, thyroid hormone increases rates of bone resorption and degradation of skin and hair.
- Thyrotropin (TSH) acts on the thyroid gland via its plasma membrane receptor and cAMP to stimulate all steps in the production of T4 and T3. These steps include iodide uptake, iodination and coupling, and retrieval from thyroglobulin. TSH also stimulates glucose oxidation, protein synthesis, and growth of epithelial cells.
|
 |
|