| In adults, the adrenal glands emerge as fairly complex endocrine structures that produce two structurally distinct classes of hormones: steroids and catecholamines. The catecholamine hormone epinephrine acts as a rapid responder to stresses such as hypoglycemia and exercise to regulate multiple parameters of physiology, including energy metabolism and cardiac output. Stress is also a major secretagogue of the longer-acting steroid hormone cortisol, which regulates glucose utilization, immune and inflammatory homeostasis, and numerous other processes. In addition, the adrenal glands regulate salt and volume homeostasis through the steroid hormone aldosterone. Finally, the adrenal gland secretes large amounts of the androgen precursor dehydroepiandrosterone sulfate (DHEAS), which plays a major role in fetoplacental estrogen synthesis and as a substrate for peripheral androgen synthesis in women.
|
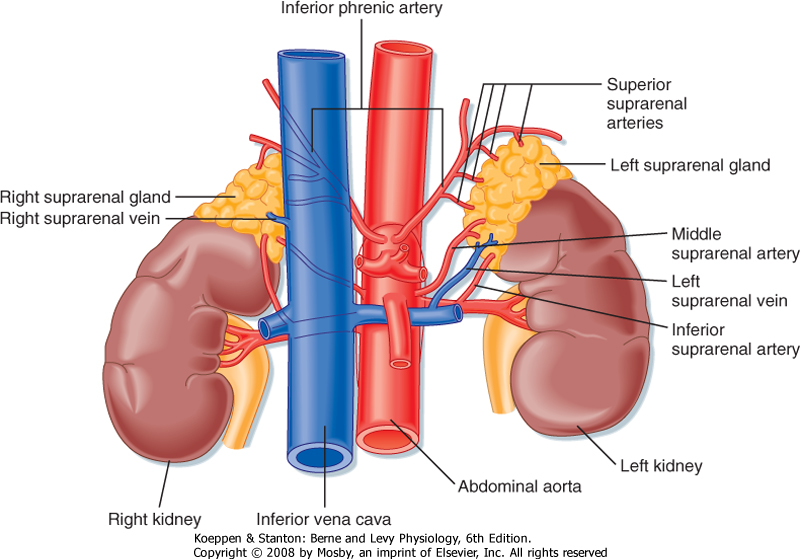
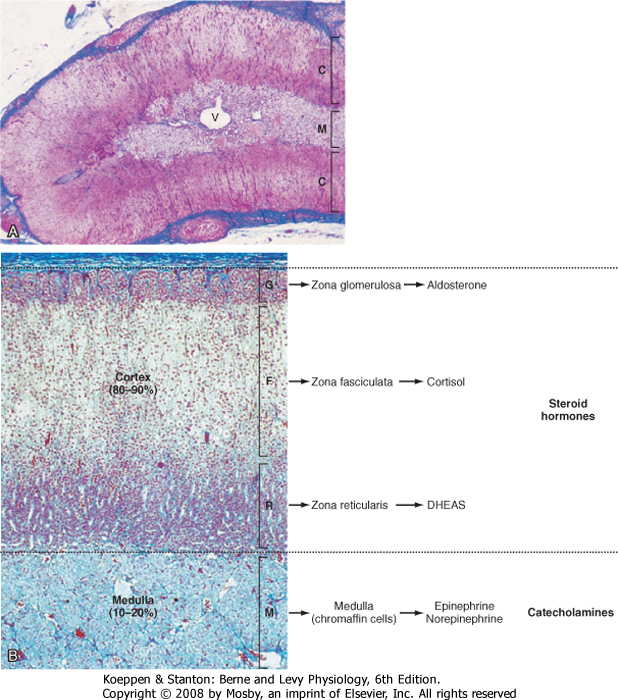
| The adrenal glands are bilateral structures located immediately above the kidneys (ad, near; renal, kidney) (Fig. 42-1). In humans, they are also referred to as the suprarenal glands because they sit on the superior pole of each kidney. The adrenal glands are similar to the pituitary in that they are derived from both neuronal tissue and epithelial (or epithelial-like) tissue. The outer portion of the adrenal gland, called the adrenal cortex (Fig. 42-2), develops from mesodermal cells in the vicinity of the superior pole of the developing kidney. These cells form cords of epithelial endocrine cells. The cells of the cortex develop into steroidogenic cells (see Chapter 37). In adults, the adrenal cortex is composed of three zones-the zona glomerulosa, the zona fasciculata, and the zona reticularis-that produce mineralocorticoids, glucocorticoids, and adrenal androgens, respectively (Fig. 42-2, B).
|
| Soon after the cortex forms, neural crest-derived cells associated with the sympathetic ganglia, called chromaffin cells, migrate into the cortex and become encapsulated by cortical cells. Thus, the chromaffin cells establish the inner portion of the adrenal gland, which is called the adrenal medulla (Fig. 42-2). The chromaffin cells of the adrenal medulla have the potential to develop into postganglionic sympathetic neurons. They are innervated by cholinergic preganglionic sympathetic neurons and can synthesize the catecholamine neurotransmitter norepinephrine from tyrosine. The enzyme phenyleth anolamine N-methyl transferase adds a methyl group to norepinephrine to produce the catecholamine hormone epinephrine, which is the primary hormonal product of the adrenal medulla (Fig. 42-2, B).
|
| Instead of being secreted near a target organ and acting as neurotransmitters, adrenomedullary catecholamines are secreted into blood and act as hormones. About 80% of the cells of the adrenal medulla secrete epinephrine, and the remaining 20% secrete norepinephrine. Although circulating epinephrine is derived entirely from the adrenal medulla, only about 30% of the circulating norepinephrine comes from the medulla. The remaining 70% is released from postganglionic sympathetic nerve terminals and diffuses into the vascular system. Because the adrenal medulla is not the sole source of catecholamine production, this tissue is not essential for life.
|
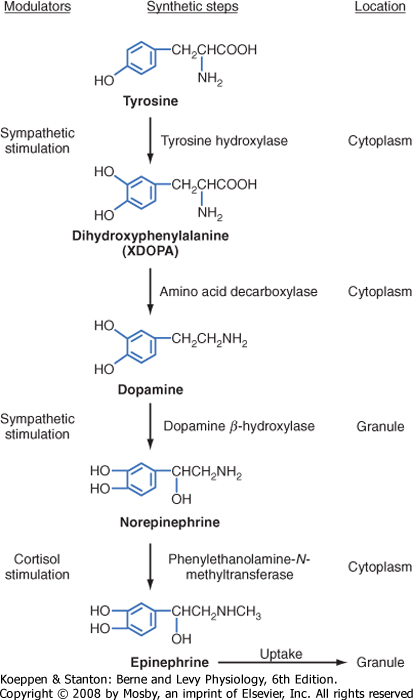
| The enzymatic steps in the synthesis of epinephrine are shown in Figure 42-4. Synthesis begins with transport of the amino acid tyrosine into the chromaffin cell cytoplasm and the subsequent hydroxylation of tyrosine by the rate-limiting enzyme tyrosine hydroxylase to produce dihydroxyphenylalanine (DOPA). DOPA is converted to dopamine by a cytoplasmic enzyme, aromatic amino acid decarboxylase, and is then transported into the secretory vesicle (also called the chromaffin granule). Within the granule, dopamine is completely converted to norepinephrine by the enzyme dopamine β-hydroxylase. In most adrenomedullary cells, essentially all of the norepinephrine diffuses out of the chromaffin granule by facilitated transport and is methylated by the cytoplasmic enzyme phenylethanolamine-N-methyltransferase to form epinephrine. Epinephrine is then transported back into the granule.
|
| page 738 |  | | page 739 |
| Figure 42-1 The adrenal glands sit on the superior poles of the kidneys and receive a rich arterial supply from the inferior, middle, and superior suprarenal arteries. The adrenals are drained by a single suprarenal vein. (Modified from Drake RL et al: Gray's Anatomy for Students. Philadelphia, Churchill Livingstone, 2005.) |
| The high local concentration of cortisol in the medulla is maintained by the vascular configuration within the adrenal gland. The outer connective tissue capsule of the adrenal gland is penetrated by a rich arterial supply coming from three main arterial branches (i.e., the inferior, middle, and superior suprarenal arteries; Fig. 42-1). These give rise to two types of blood vessels that carry blood from the cortex to the medulla (Fig. 42-3): (1) relatively few medullary arterioles, which provide high oxygen- and nutrient-laden blood directly to the medullary chromaffin cells, and (2) relatively numerous cortical sinusoids, into which cortical cells secrete steroid hormones (including cortisol). Both vessel types fuse to give rise to the medullary plexus of vessels that ultimately drains into a single suprarenal vein. Thus, secretions of the adrenal cortex percolate through the chromaffin cells and bathe them in high concentrations of cortisol before leaving the gland and entering the inferior vena cava. Cortisol inhibits neuronal differentiation of the medullary cells, so they fail to form dendrites and axons. Additionally, cortisol induces expression of the enzyme phenylethanolamine-N-methyltransferase (PNMT), which converts norepinephrine to epinephrine (Fig. 42-4). Glucocorticoid receptor (see later) knockout mice have an enlarged cortex, but the size of the medulla is decreased and PNMT activity is undetectable. |
 |
| Secretion of epinephrine and norepinephrine from the adrenal medulla is regulated primarily by descending sympathetic signals in response to various forms of stress, including exercise, hypoglycemia, and hemorrhagic
hypovolemia (Fig. 42-5). The primary autonomic centers that initiate sympathetic responses reside in the hypothalamus and brainstem, and they receive input from the cerebral cortex, the limbic system, and other regions of the hypothalamus and brainstem.
|
| The chemical signal for secretion of catecholamine from the adrenal medulla is acetylcholine (ACh), which is secreted from preganglionic sympathetic neurons and binds to nicotinic receptors on chromaffin cells (Fig. 42-5). ACh increases the activity of the rate-limiting enzyme tyrosine hydroxylase in chromaffin cells (Fig. 42-4). It also increases the activity of dopamine β-hydroxylase and stimulates exocytosis of the chromaffin granules. Synthesis of epinephrine and norepinephrine is closely coupled to secretion so that levels of intracellular catecholamines do not change significantly, even in the face of changing sympathetic activity.
|
| Mechanism of Action of Catecholamines
|
| Adrenergic receptors are generally classified as α- and β-adrenergic receptors, with the α-adrenergic receptors further divided into α1 and α2 receptors and the β-adrenergic receptors divided into β1, β2, and β3 receptors (Table 42-1). These receptors can be characterized according to (1) the relative potency of endogenous and pharmacological agonists and antagonists. Epinephrine and norepinephrine are potent agonists for α receptors and for β1 and β3 receptors, whereas epinephrine is more potent than norepinephrine for β2 receptors. A large number of synthetic selective and nonselective adrenergic agonists and antagonists now exist. (2) Downstream signaling pathways. Table 42-1 shows the primary pathways that are coupled to the different adrenergic receptors. This is an oversimplification because differences in signaling pathways for a given receptor have been linked to the duration of agonist exposure and cell type. (3) Location and relative density of receptors. Importantly, different receptor types predominate in different tissues. For example, although both α and β receptors are expressed by pancreatic islet beta cells, the predominant response to a sympathetic discharge is mediated by α2 receptors.
|
| page 739 |  | | page 740 |
| Figure 42-2 Histology of the adrenal gland. A, Low magnification illustrating the outer cortex (C) and inner medulla (M; note the central vein [V]). B, Higher magnification clearly illustrating the zonation of the cortex. The corresponding endocrine function and the different zones of the cortex and the medulla are noted. (From Young B et al: Wheater's Functional Histology, 5th ed. Philadelphia, Churchill Livingstone, 2006.) |
|
Table 42-1.
Adrenergic Receptors |
| Receptor Type | Primary Mechanism of Action | Examples of Tissue Distribution | Examples of Action |
| α1 | ↑ IP3 and Ca++, DAG | Sympathetic postsynaptic nerve terminals | Increase vascular smooth muscle contraction |
| α2 | ↓ cAMP | Sympathetic presynaptic nerve terminals; beta cell of pancreatic islets | Inhibit norepinephrine release; inhibit insulin release |
| β1 | ↑ cAMP | Heart | Increase cardiac output |
| β2 | ↑ cAMP | Liver; smooth muscle of vasculature, bronchioles, and uterus | Increase hepatic glucose output; decrease contraction of blood vessels, bronchioles, and uterus |
| β3 | ↑ cAMP | Liver; adipose tissue | Increase hepatic glucose output; increase lipolysis |
|
| page 740 |  | | page 741 |
| Figure 42-3 Blood flow through the adrenal gland. Capsular arteries give rise to sinusoidal vessels that carry blood centripetally through the cortex to the medulla. (Modified from Young B et al: Wheater's Functional Histology, 5th ed. Philadelphia, Churchill Livingstone, 2006.) |
| Physiological Actions of Adrenomedullary Catecholamines
|
| Because the adrenal medulla is directly innervated by the autonomic nervous system, adrenomedullary responses are very rapid. Furthermore, because of the involvement of several centers in the central nervous system (CNS), most notably the cerebral cortex, adrenomedullary responses can precede onset of the actual stress (i.e., they can be anticipated) (Fig. 42-5). In many cases, the adrenomedullary output, which is primarily epinephrine, is coordinated with sympathetic nervous activity, as determined by the release of norepinephrine from postganglionic sympathetic neurons. However, some stimuli (e.g., hypoglycemia) evoke a stronger adrenomedullary than sympathetic nervous response, and vice versa.
|
|
Table 42-2.
Some Actions of Catecholamine Hormones |
| β: Epinephrine > Norepinephrine | α: Norepinephrine > Epinephrine |
| ↑ Glycogenolysis | ↑ Gluconeogenesis (α1) |
| ↑ Gluconeogenesis (β2) | ↑ Glycogenolysis (α1) |
| ↑ Lipolysis (β3) (β2) | |
| ↑ Calorigenesis (β1) | |
| ↓ Glucose utilization | |
| ↑ Insulin secretion (β2) | ↓ Insulin secretion (α2) |
| ↑ Glucagon secretion (β2) | |
| ↑ Muscle K+ uptake (β2) | ↑ Cardiac contractility (α1) |
| ↑ Cardiac contractility (β1) | |
| ↑ Heart rate (β1) | |
| ↑ Conduction velocity (β1) | |
| ↑ Arteriolar dilation: ↓ BP (β2) (muscle) | ↑ Arteriolar vasoconstriction; ↑BP (α1) (splanchnic, renal, cutaneous, genital) |
| ↑ Muscle relaxation (β2) | ↑ Sphincter contraction (α1) |
| Gastrointestinal | Gastrointestinal |
| Urinary | Urinary |
| Bronchial | ↑Platelet aggregation (α2) |
| | ↑Sweating ("adrenergic") |
| | ↑Dilation of pupils (α1) |
|
| page 741 |  | | page 742 |
Many organs and tissues are affected by a sympathoadrenal response (Table 42-2). An informative example of the major physiological roles of catecholamines is the sympathoadrenal response to exercise. Exercise is similar to the "fight or flight" response, but without the subjective element of fear, and involves a greater adrenomedullary response (i.e., endocrine role of epinephrine) than a sympathetic nervous response (i.e., neurotransmitter role of norepinephrine). The overall goal of the sympathoadrenal system during exercise is to meet the increased energy demands of skeletal and cardiac muscle while maintaining sufficient oxygen and glucose supply to the brain. The response to exercise includes the following major physiological actions of epinephrine (Fig. 42-6):
- Increased blood flow to muscles is achieved by the integrated action of norepinephrine and epinephrine on the heart, veins and lymphatics, and nonmuscular (e.g., splanchnic) and muscular arteriolar beds.
- Epinephrine promotes glycogenolysis in muscle. Exercising muscle can also utilize free fatty acids
(FFAs), and epinephrine and norepinephrine promote lipolysis in adipose tissue. The actions just described increase circulating levels of lactate and glycerol, which can be used by the liver as gluconeogenic substrates to increase glucose. Epinephrine increases blood glucose by increasing hepatic glycogenolysis and gluconeogenesis. The promotion of lipolysis in adipose tissue is also coordinated with an epinephrine-induced increase in hepatic ketogenesis. Finally, the effects of catecholamines on metabolism are reinforced by the fact that they stimulate glucagon secretion (β2 receptors) and inhibit insulin secretion (α2 receptors). Efficient production of ATP during normal exercise (i.e., a 1-hour workout) also requires efficient exchange of gases with an adequate supply of oxygen to exercising muscle. Catecholamines promote this by relaxation of bronchiolar smooth muscle.
- Catecholamines decrease energy demand by visceral smooth muscle. In general, a sympathoadrenal response decreases overall motility of the smooth muscle in the gastrointestinal (GI) and urinary tracts, thereby conserving energy where it is not needed.
|
|
| Figure 42-4 Steps in the synthesis of catecholamines. |
| Figure 42-5 Stimuli that enhance the secretion of catecholamines. |
| Metabolism of Catecholamines
|
| Two primary enzymes are involved in the degradation of catecholamines: monoamine oxidase (MAO) and catechol-O-methyltransferase (COMT) (Fig. 42-7). The neurotransmitter norepinephrine is degraded by MAO and COMT after uptake into the presynaptic terminal. This mechanism is also involved in the catabolism of circulating adrenal catecholamines. However, the predominant fate of adrenal catecholamines is methylation by COMT in nonneuronal tissues such as the liver and kidney. Urinary vanillylmandelic acid (VMA) and metanephrine are sometimes used clinically to assess the level of catecholamine production in a patient. Much of the urinary VMA and metanephrine is derived from neuronal rather than adrenal catecholamines.
|
| page 742 |  | | page 743 |
| Figure 42-6 Some of the individual actions of catecholamines that contribute to the integrated sympathoadrenal response to exercise. (Modified from Porterfield SP, White BA: Endocrine Physiology, 3rd ed. Philadelphia, Mosby, 2007.) |
| Figure 42-7 Degradative metabolism of catecholamines. MAO stimulates deamination; COMT stimulates methylation. |
| page 743 |  | | page 744 |
| Pheochromocytoma is a tumor of chromaffin tissue that produces excessive quantities of catecholamines. These are commonly adrenal medullary tumors, but they can occur in other chromaffin cells of the autonomic nervous system. Although pheochromocytomas are not common tumors, they are the most common cause of hyperfunctioning of the adrenal medullary. The catecholamine most frequently elevated in pheochromocytoma is norepinephrine. For unknown reasons, the symptoms of excessive catecholamine secretion are often sporadic rather than continuous. Symptoms include hypertension, headaches (from hypertension), sweating, anxiety, palpitations, and chest pain. In addition, patients with this disorder may show orthostatic hypotension (despite the tendency for hypertension). This occurs because hypersecretion of catecholamines can decrease the postsynaptic response to norepinephrine as a result of down-regulation of the receptors (see Chapter 3). Consequently, the baroreceptor response to blood shifts that occurs on standing is blunted. |
 |
|
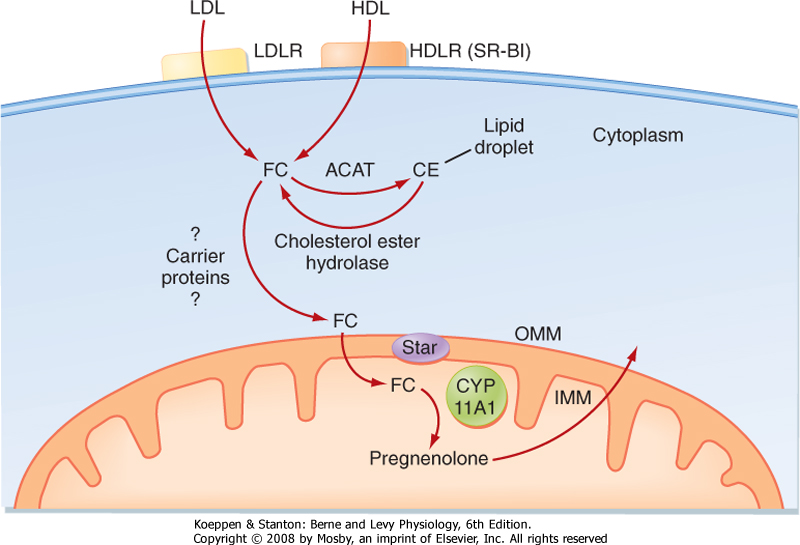
| Figure 42-8 Events involved in the first reaction in the steroidogenic pathway (conversion of cholesterol to pregnenolone) in zona fasciculata cells. ACAT, acyl CoA : cholesterol acyltransferase; CE, cholesterol esters; FC, free cholesterol; HDLR, high-density lipoprotein receptor (also called the scavenger receptor BI [SR-BI]); IMM, inner mitochondrial membrane; LDLR, low-density lipoprotein receptor; OMM, outer mitochondrial membrane; StAR, steroidogenic acute regulatory protein. (Modified from Porterfield SP, White BA: Endocrine Physiology, 3rd ed. Philadelphia, Mosby, 2007.) |
| The zona fasciculata produces the glucocorticoid hormone cortisol. This zone is an actively steroidogenic tissue composed of straight cords of large cells. These cells have a "foamy" cytoplasm because they are filled with lipid droplets that represent stored cholesterol esters. These cells make some cholesterol de novo but also import cholesterol from blood in the form of low-density lipoprotein (LDL) and high-density
lipoprotein (HDL) particles. Free cholesterol is then esterified and stored in lipid droplets (Fig. 42-8). The stored cholesterol is continually turned back into free cholesterol by a cholesterol ester hydrolyase, a process that is increased in response to the stimulus of cortisol synthesis (e.g., adrenocorticotropic hormone [ACTH]-see later). In the zona fasciculata, cholesterol is converted sequentially to pregnenolone, progesterone, 17-hydroxyprogesterone, 11-deoxycortisol, and cortisol (Figs. 42-9 and 42-10). A parallel pathway in the zona fasciculata involves the conversion of progesterone to 11-deoxycorticosterone (DOC) and then to corticosterone (Fig. 42-10, C). This pathway is minor in humans, but in the absence of active
CYP11B1 (11-hydroxylase activity), the production of DOC is significant. Because DOC acts as a weak mineralocorticoid (Table 42-3), elevated levels of DOC cause hypertension.
|
| Transport and Metabolism of Cortisol
|
| Cortisol is transported in blood predominantly bound to corticosteroid-binding globulin [CBG] (also called transcortin), which binds about 90%, and albumin, which binds 5% to 7% of the circulating hormone. The liver is the predominant site of steroid inactivation. It both inactivates cortisol and conjugates active and inactive steroids with glucuronide or sulfate so that they can be excreted more readily by the kidney. The circulating half-life of cortisol is about 70 minutes.
|
|
Table 42-3.
Relative Glucocorticoid and Mineralocorticoid Potency of Natural Corticosteroids and Some Synthetic Analogues in Clinical Use* |
| | Glucocorticoid | Mineralocorticoid |
| Corticosterone | 0.5 | 1.5 |
| Prednisone (1.2 double bond) | 4 | <0.1 |
| 6α-Methylprednisone (Medrol) | 5 | <0.1 |
| 9α-Fluoro-16α-hydroxyprednisolone (triamcinolone) | 5 | <0.1 |
| 9α-Fluoro-16α-methylprednisolone (dexamethasone) | 30 | <0.1 |
| Aldosterone | 0.25 | 500 |
| Deoxycorticosterone | 0.01 | 30 |
| 9α-Fluorocortisol | 10 | 500 |
*All values are relative to the glucocorticoid and mineralocorticoid potencies of cortisol, which have each been set at 1.0 arbitrarily. Cortisol actually has only 1/500 the potency of the natural mineralocorticoid aldosterone.
|
| page 744 |  | | page 745 |
| Free cholesterol is modified by five reactions within a steroidogenic pathway to form cortisol (Fig. 42-8). However, cholesterol is stored in the cytoplasm, and the first enzyme of the pathway, CYP11A1, is located on the inner mitochondrial membrane (Fig. 42-9). Thus, the rate-limiting reaction in steroidogenesis is the transfer of cholesterol from the outer to the inner mitochondrial membrane. Although several proteins appear to be involved, one protein, called steroidogenic acute regulatory protein (StAR protein), is indispensable in the process of transporting cholesterol to the inner mitochondrial membrane (Fig. 42-8). StAR protein is short-lived and rapidly activated posttranslationally (phosphorylation) and transcriptionally by pituitary tropic hormones. In patients with inactivating mutations in StAR protein, cells of the zona fasciculata become excessively laden with lipid ("lipoid") because cholesterol cannot be accessed by CYP11A1 within the mitochondria and used for cortisol synthesis. Moreover, these individuals cannot form sex steroid hormones. The placenta does not express StAR, so these individuals have normal placental steroid production in utero. |
 |
|
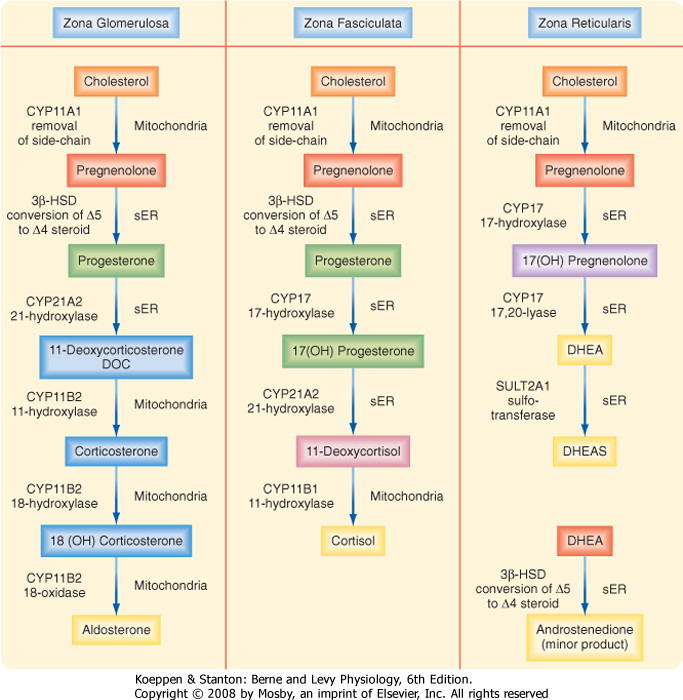
| Figure 42-9 Summary of the steroidogenic pathways for each of the three zones of the adrenal cortex. The enzymatic reactions are color-coded across zones. sER, smooth endoplasmic reticulum. (Modified from Porterfield SP, White BA: Endocrine Physiology, 3rd ed. Philadelphia, Mosby, 2007.) |
| page 745 |  | | page 746 |
| Steroidogenic enzymes fall into two superfamilies. Most belong to the cytochrome P-450 monooxidase gene family and are thus referred to as CYPs. These enzymes are located either in the inner mitochondrial matrix, where they use molecular oxygen and a flavoprotein electron donor, or in the smooth endoplasmic reticulum, where they use a different flavoprotein for electron transfer. Different CYP enzymes act as hydroxylases, lyases (desmolases), oxidases, or aromatases. Two of these enzymes have multiple functions. CYP17 has both a 17-hydroxylase function and a 17,20-lyase (desmolase) function. CYP11B2, also called aldosterone synthase, has three functions: 11-hydroxylase, 18-hydroxylase, and 18-oxidase. |
| The other enzymes involved in steroidogenesis belong to three hydroxysteroid dehydrogenase (HSD) families. 3β-HSDs have two isoforms that convert the hydroxyl group on carbon 3 of the cholesterol ring to a ketone and shift the double bond from the 5-6 (Δ5) position to the 4-5 (Δ4) position. All active steroid hormones must be converted to Δ4 structures by 3β-HSD. The 17β-HSDs have at least five members and can act as either oxidases or reductases. 17β-HSDs primarily act on sex steroids and can be activating or deactivating. Finally, the 11β-HSDs have two isoforms that catalyze the interchange between cortisol (active) and cortisone (inactive). |
 |

|
| Figure 42-10 A, Reaction 1, catalyzed by CYP11A1, in making cortisol. B, Reactions 2a/b and reactions 3a/b, involving CYP17 (17-hydroxylase function) and 3β-hydroxysteroid dehydrogenase (3β-HSD), in making cortisol. This figure shows the Δ5 versus Δ4 pathway. C, Reactions 4 and 5, involving CYP21B and CYP11B1, in which the last two steps in the synthesis of cortisol are carried out. Also shown is the minor pathway leading to the synthesis of corticosterone in the zona fasciculata. (Modified from Porterfield SP, White BA: Endocrine Physiology, 3rd ed. Philadelphia, Mosby, 2007.) |
| page 746 |  | | page 747 |
| Cortisol is reversibly inactivated by conversion to cortisone. This action is catalyzed by the enzyme
11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2). The inactivation of cortisol by 11β-HSD2 is reversible in that another enzyme, 11β-HSD1, converts cortisone back to cortisol. This conversion occurs in tissues expressing the glucocorticoid receptor (GR), including liver, adipose tissue, and the CNS, as well as in skin (which is why cortisone-based creams can be applied to skin to stop inflammation).
|
| Mechanism of Action of Cortisol
|
Cortisol acts primarily through the glucocorticoid receptor, which regulates gene transcription (see Chapter 3). In the absence of hormone, the GR resides in the cytoplasm in a stable complex with several molecular chaperones, including heat shock proteins and cyclophilins. Cortisol-GR binding promotes dissociation of the chaperone proteins, followed by
- Rapid translocation of the cortisol-GR complex into the nucleus
- Dimerization and binding to glucocorticoid response elements (GREs) near the basal promoters of cortisol-regulated genes
- Recruitment of coactivator proteins and assembly of general transcription factors leading to increased transcription of the targeted genes.
|
| page 747 |  | | page 748 |
| Glucocorticoids can also repress gene transcription. In some cases, the GR interacts with other transcription factors, such as the proinflammatory NF-κB transcription factor, and interferes with their ability to
activate gene expression. In other cases, GR binds to "negative GREs" and recruits corepressor proteins.
|
| Physiological Actions of Cortisol
|
| Cortisol has a broad range of action and is often characterized as a "stress hormone." In general, cortisol maintains blood glucose levels, CNS function, and cardiovascular function during fasting and increases blood glucose during stress at the expense of muscle protein. Cortisol protects the body against the self-injurious effects of unbridled inflammatory and immune responses. Cortisol also partitions energy to cope with stress by inhibiting reproductive function. As stated later, cortisol has several other effects on bone, skin, connective tissue, the GI tract, and the developing fetus that are independent of its stress-related functions.
|
| Figure 42-11 Metabolic actions of cortisol (integrated with catecholamines and glucagon) in response to stress (upper panel) and contrasted to the actions of chronically elevated cortisol (integrated with insulin) in an otherwise healthy individual (lower panel). (Modified from Porterfield SP, White BA: Endocrine Physiology, 3rd ed. Philadelphia, Mosby, 2007.) |
| As the term glucocorticoid implies, cortisol is a steroid hormone from the adrenal cortex that regulates blood glucose. It increases blood glucose by stimulating gluconeogenesis (Fig. 42-11). Cortisol enhances gene expression of the hepatic
gluconeogenic enzymes phosphoenolpyruvate carboxykinase (PEPCK), fructose-1,6-bisphosphatase, and glucose-6-phosphatase (G6Pase). Cortisol also decreases Glut4-mediated glucose uptake in skeletal muscle and adipose tissue. During the interdigestive period (low insulin-glucagon ratio), cortisol promotes glucose sparing by potentiating the effects of catecholamines on lipolysis, thereby making FFAs available as energy sources. Cortisol inhibits protein synthesis and increases proteolysis, especially in skeletal muscle, thereby providing a rich source of carbon for hepatic gluconeogenesis.
|
| page 748 |  | | page 749 |
| Figure 42-11 also contrasts the normal role of cortisol in response to stress and the effects of chronically elevated cortisol as a result of pathological conditions. As discussed later, there are important differences in the overall metabolic effects of cortisol between these two states, particularly with respect to lipid metabolism. During stress, cortisol synergizes with catecholamines and glucagon to promote a lipolytic, gluconeogenic, ketogenic, and glycogenolytic metabolic response while synergizing with
catecholamines to promote an appropriate cardiovascular response. During chronically elevated cortisol secondary to pathological overproduction, cortisol synergizes with insulin in the context of elevated levels of glucose (from increased appetite) and hyperinsulinemia (from elevated glucose and glucose intolerance) to promote lipogenesis and truncal (abdominal, visceral) adiposity.
|
| Cortisol reinforces its effects on blood glucose by its positive effects on the cardiovascular system. Cortisol has permissive actions on catecholamines and thereby contributes to cardiac output and blood pressure. Cortisol stimulates erythropoietin synthesis and hence increases red blood cell production. Anemia occurs when cortisol is deficient, and polycythemia occurs when cortisol levels are excessive.
|
| Antiinflammatory and Immunosuppressive Actions
|
| Inflammation and immune responses are often part of the response to stress. However, inflammation and immune responses have the potential for significant harm and may cause death if they are not held in homeostatic balance. As a stress hormone, cortisol plays an important role in maintaining immune homeostasis. Cortisol, along with epinephrine and norepinephrine, represses the production of proinflammatory cytokines and stimulates the production of antiinflammatory cytokines
|
| The inflammatory response to injury consists of local dilation of capillaries and increased capillary permeability with resultant local edema and accumulation of white blood cells. These steps are mediated by prostaglandins, thromboxanes, and leukotrienes. Cortisol inhibits phospholipase A2, a key enzyme in prostaglandin, leukotriene, and thromboxane synthesis. Cortisol also stabilizes lysosomal membranes, thereby decreasing release of the proteolytic enzymes that augment local swelling. In response to injury, leukocytes normally migrate to the site of injury and leave the vascular system. These effects are inhibited by cortisol, as is the phagocytic activity of neutrophils, although release of neutrophils from bone marrow is stimulated. Analogues of glucocorticoid are frequently used pharmacologically because of their antiinflammatory properties.
|
| Cortisol inhibits the immune response, and for this reason glucocorticoid analogues have been used as immunosuppressants in organ transplants. High cortisol levels decrease the number of circulating T lymphocytes (particularly helper T lymphocytes) and reduce their ability to migrate to the site of antigenic stimulation. Glucocorticoids promote atrophy of the thymus and other lymphoid tissue. Although corticosteroids inhibit cellular-mediated immunity, antibody production by B lymphocytes is not impaired.
|
| Effects of Cortisol on the Reproductive Systems
|
| Reproduction exacts a considerable anabolic cost on the organism. In humans, reproductive behavior and function are dampened in response to stress. Cortisol decreases the function of the reproductive axis at the hypothalamic, pituitary, and gonadal levels.
|
| Effects of Cortisol on Bone
|
| Glucocorticoids increase bone resorption. They have multiple actions that alter bone metabolism. Glucocorticoids decrease intestinal Ca++ absorption and renal Ca++ reabsorption. Both mechanisms serve to lower serum [Ca++]. As serum [Ca++] drops, secretion of parathyroid hormone (PTH) increases, and PTH mobilizes Ca++ from bone by stimulating resorption of bone. In addition to this action, glucocorticoids directly inhibit osteoblast bone-forming functions (see Chapter 39). Although glucocorticoids are useful for treating the inflammation associated with arthritis, excessive use will result in bone loss (osteoporosis).
|
| Actions of Cortisol on Connective Tissue
|
| Cortisol inhibits fibroblast proliferation and collagen formation. In the presence of excessive amounts of cortisol, the skin thins and is more readily damaged. The connective tissue support of capillaries is impaired, and capillary injury, or bruising, is increased.
|
| Actions of Cortisol on the Kidney
|
| Cortisol inhibits the secretion and action of antidiuretic hormone (ADH), and thus it is an ADH antagonist. In the absence of cortisol, the action of ADH is potentiated, which makes it difficult to increase free water clearance in response to a water load and increases the likelihood of water intoxication. Although cortisol binds to the mineralocorticoid receptor with high affinity, this action is normally blocked by inactivation of cortisol to cortisone by the enzyme 11β-HSD2. However, the mineralocorticoid activity (i.e., renal Na+ and H2O retention, K+ and H+ excretion) of cortisol depends on the relative amount of cortisol (or synthetic glucocorticoids) and the activity of 11β-HSD2. Certain agents (such as compounds in black licorice) inhibit 11β-HSD2 and thereby increase the mineralocorticoid activity of cortisol. Cortisol increases the glomerular filtration rate by both increasing cardiac output and acting directly on the kidney.
|
| Actions of Cortisol on Muscle
|
| When cortisol levels are excessive, muscle weakness and pain are common symptoms. The weakness has multiple origins. In part, it is a result of the excessive proteolysis that cortisol produces. High cortisol levels can result in hypokalemia (via mineralocorticoid actions), which can produce muscle weakness because it hyperpolarizes and stabilizes the muscle cell membrane and thus makes stimulation more difficult.
|
| Actions of Cortisol on the Gastrointestinal Tract
|
| Cortisol exerts a trophic effect on the GI mucosa. In the absence of cortisol, GI motility decreases, GI mucosa degenerates, and GI acid and enzyme production decreases. Because cortisol stimulates appetite, hypercortisolism is frequently associated with weight gain. The cortisol-mediated stimulation of gastric acid and pepsin secretion increases the risk for development of ulcers.
|
| Psychological Effects of Cortisol
|
| page 749 |  | | page 750 |
| Psychiatric disturbances are associated with either excessive or deficient levels of corticosteroids.
Excessive corticosteroids can initially produce a feeling of well-being, but continued excessive exposure eventually leads to emotional lability and depression. Frank psychosis can occur with either excessive or deficient hormone. Cortisol increases the tendency for insomnia and decreases rapid eye movement (REM) sleep. People who are deficient in corticosteroids tend to be depressed, apathetic, and irritable.
|
| Effects of Cortisol during Fetal Development
|
| Cortisol is required for normal development of the CNS, retina, skin, GI tract, and lungs. The best studied system is the lungs, in which cortisol induces differentiation and maturation of type II alveolar cells. During late gestation these cells produce surfactant, which reduces surface tension in the lungs and thus allows the onset of breathing at birth.
|
| Regulation of Cortisol Production
|
| Cortisol production by the zona fasciculata is regulated by a standard hypothalamus-pituitary-adrenal axis involving corticotropin-releasing hormone (CRH), ACTH, and cortisol (see Chapter 40). The hypothalamus and pituitary stimulate cortisol production, and cortisol negatively feeds back on the hypothalamus and pituitary to maintain its set point. Both neurogenic (e.g., fear) and systemic (e.g., hypoglycemia, hemorrhage, cytokines) forms of stress stimulate release of CRH. CRH is also under strong diurnal rhythmic regulation emerging from the suprachiasmatic nucleus such that cortisol levels surge during the early predawn and morning hours and then continually decline throughout the day and evening. CRH acutely stimulates release of ACTH and chronically increases proopiomelanocortin (POMC) gene expression and corticotrope hypertrophy and proliferation. Some parvicellular neurons coexpress CRH and ADH, which potentiates the actions of CRH.
|

|
| Figure 42-12 Overview of the actions of ACTH on target adrenocortical cells. Note that the major second messenger, cAMP, activates immediate protein mediators and also induces the production of later protein mediators. HDL, high-density lipoprotein; LDL, low-density lipoprotein. |
ACTH binds to the melanocortin 2 receptor (MC2R) located on cells in the zona fasciculata (Fig. 42-12). The effects of ACTH can be subdivided into three phases:
- The acute effects of ACTH occur within minutes. Cholesterol is rapidly mobilized from lipid droplets by posttranslational activation of cholesterol ester hydrolase and transported to the outer mitochondrial membrane. ACTH both rapidly increases steroidogenic acute regulatory (StAR) protein gene expression and activates StAR protein through protein kinase A (PKA)-dependent phosphorylation. Collectively, these acute actions of ACTH increase pregnenolone levels.
- The chronic effects of ACTH occur over a period of several hours. These effects involve increasing transcription of the genes encoding the steroidogenic enzymes and their coenzymes. ACTH also increases expression of the LDL receptor and scavenger receptor BI (SR-BI; the HDL receptor).
- The trophic actions of ACTH on the zona fasciculata and zona reticularis occur over a period of weeks and months. This effect is exemplified by atrophy of the zona fasciculata in patients receiving therapeutic (i.e., supraphysiological) levels of glucocorticoid analogues for at least 3 weeks. Under these conditions, the exogenous corticosteroids completely repress CRH and ACTH production, thereby resulting in atrophy of the zona fasciculata and a decline in endogenous cortisol production (Fig. 42-13). At the end of therapy, these patients need to be slowly weaned off exogenous glucocorticoids to allow the hypothalamus-pituitary-adrenal axis to reestablish itself and the zona fasciculata to enlarge and produce adequate amounts of cortisol.
|
| page 750 |  | | page 751 |
| Cortisol inhibits both POMC gene expression at the corticotropes and pro-CRH gene expression at the hypothalamus. However, intense stress can override the negative-feedback effects of cortisol at the hypothalamus and reset the "set point" at a higher level.
|
| The innermost zone, the zona reticularis, begins to appear after birth at about 5 years of age. Adrenal androgens, especially DHEAS, the main product of the zona reticularis, become detectable in the circulation at about 6 years of age. This onset of adrenal androgen production is called adrenarche, and it contributes to the appearance of axillary and pubic hair at about age 8. DHEAS levels continue to increase, peak during the mid-twenties, and then progressively decline with age.
|
| Androgen Synthesis by the Zona Reticularis
|
| Figure 42-13 Comparison of a normal hypothalamus-pituitary-adrenal (HPA) axis to a quiescent HPA axis in individual receiving exogenous glucocorticoid therapy. The latter causes the zona fasciculata to atrophy after 3 weeks, thus requiring a careful withdrawal regimen to allow rebuilding of the adrenal tissue before total cessation of exogenous corticosteroid administration. (Modified from Porterfield SP, White BA: Endocrine Physiology, 3rd ed. Philadelphia, Mosby, 2007.) |
| The zona reticularis differs from the zona fasciculata in several important ways with respect to steroidogenic enzyme activity (Fig. 42-9). First, 3β-HSD is expressed at much lower levels in the zona reticularis
than in the zona fasciculata; thus, the "Δ5 pathway" predominates in the zona reticularis. Second, the zona reticularis expresses cofactors or conditions that enhance the 17,20-lyase function of CYP17, thereby generating the 19-carbon androgen precursor molecule dehydroepiandrosterone (DHEA) from 17-hydroxypregnenolone. Additionally, the zona reticularis expresses DHEA sulfotransferase (SULT2A1 gene), which converts DHEA into DHEAS (Fig. 42-14). A limited amount of the Δ4 androgen androstenedione is also made in the zona reticularis. Although small amounts of potent androgens (e.g., testosterone) or 18-carbon estrogens are normally produced by the human adrenal cortex, most active sex steroids are produced primarily from peripheral conversion of DHEAS and androstenedione.
|
| Metabolism and Fate of DHEAS and DHEA
|
| DHEAS can be converted back to DHEA by peripheral sulfatases, and DHEA and androstenedione can be converted to active androgens (testosterone, dihydrotestosterone) peripherally in both sexes. DHEA binds to albumin and other globulins in blood with low affinity, so it is excreted efficiently by the kidney. The half-life of DHEA is 15 to 30 minutes. In contrast, DHEAS binds to albumin with very high affinity and has a half-life of 7 to 10 hours.
|
| Physiological Actions of Adrenal Androgens
|
| In men, the contribution of adrenal androgens to active androgens is negligible. However, in women, the adrenal contributes to about 50% of circulating active androgens, which are required for the growth of axillary and pubic hair and for libido.
|
| Apart from providing androgen precursors, it is not clear what other role or roles, if any, that the zona reticularis plays in adult humans. DHEAS is the most abundant circulating hormone in young adults. It increases steadily until it peaks in the mid-twenties and then steadily declines thereafter. Thus, there has been considerable interest in the possible role of DHEAS in the aging process. However, the function of this abundant steroid in young adults and the potential impact of its gradual disappearance on aging are still poorly understood. It should be noted that the age-related decline in DHEA and DHEAS has led to the popular use of these steroids as dietary supplements, even though recent studies indicate no beneficial effects.
|
| During adrenal androgen excess (e.g., adrenal tumor, Cushing's syndrome, congenital adrenal hyperplasia), masculinization of women can occur. This involves masculinization of the external genitalia (e.g., enlarged clitoris) in utero and excessive facial and body hair (called hirsutism) and acne in adult women. Excessive adrenal androgens also appear to play a role in ovarian dysovulation (i.e., polycystic ovarian syndrome). |
| page 751 |  | | page 752 |
| Figure 42-14 Steroidogenic pathways in the zona reticularis. The first common reaction in the pathway, conversion of cholesterol to pregnenolone by CYP11A1, is not shown. Expression of 3β-hydroxysteroid dehydrogenase (3β-HSD) is relatively low in the zona reticularis, so androstenedione is a minor product in comparison to DHEA and DHEAS. The zona reticularis also makes a small amount of testosterone and estrogens (not shown). (Modified from Porterfield SP, White BA: Endocrine Physiology, 3rd ed. Philadelphia, Mosby, 2007.) |
| page 752 |  | | page 753 |
| Figure 42-15 The "loophole" in the hypothalamus-pituitary-adrenal axis. ACTH stimulates the production of both cortisol and adrenal androgens, but only cortisol negatively feeds back on ACTH and CRH. Thus, if cortisol production is blocked (i.e., CYP11B1 deficiency), ACTH levels increase, along with adrenal androgens. (Modified from Porterfield SP, White BA: Endocrine Physiology, 3rd ed. Philadelphia, Mosby, 2007.) |
| A crucial clinical aspect of regulation of the zona reticularis is that neither adrenal androgens nor their more potent metabolites (e.g., testosterone, dihydrotestosterone, estradiol-17β) negatively feed back on ACTH or CRH (Fig. 42-15). This means that an enzymatic defect associated with the synthesis of cortisol (e.g., CYP21B deficiency) is associated with a dramatic increase in both ACTH (no negative feedback from cortisol) and adrenal androgens (because of the elevated ACTH). It is this "loophole" in the hypothalamus-pituitary-adrenal axis that gives rise to congenital adrenal hyperplasia. |
| Regulation of Zona Reticularis Function
|
| ACTH is the primary regulator of the zona reticularis. Both DHEA and androstenedione display the same diurnal rhythm as cortisol (DHEAS does not because of its long circulating half-life). Moreover, the zona reticularis shows the same atrophic changes as the zona fasciculata in conditions typified by little or no ACTH. However, other factors must regulate adrenal androgen function. Adrenarche occurs in the face of constant ACTH and cortisol levels, and the rise and decline of DHEAS is not associated with a similar pattern of ACTH or cortisol production. However, the other factors, whether extraadrenal or intraadrenal, remain unknown.
|
| The thin, outermost zone of the adrenal, the zona glomerulosa, produces the mineralocorticoid aldosterone, which regulates salt and volume homeostasis (see Chapter 34). The zona glomerulosa is minimally influenced by ACTH. Rather, it is regulated primarily by the renin-angiotensin system, plasma [K+], and atrial natriuretic peptide (ANP).
|
| CYP11B1 and CYP11B2 are located on chromosome 8 in humans, display 95% similarity, and are separated from each other by only about 50 kilobases. This increases the possibility of uneven crossing over during gametogenesis, with the formation of hybrid genes. In one case, the promoter region and 5' end of the CYP11B1 gene is fused to the 3' end of the CYP11B2 gene. This arrangement leads to aldosterone synthase being expressed in the zona fasciculata and reticularis under the control of ACTH. Because aldosterone is no longer under feedback control by the renin-angiotensin system (see Chapter 34), aldosterone levels are high, and hypertension ensues. This form of primary aldosteronism is called glucocorticoid-remediable aldosteronism, and it is inherited in an autosomal dominant manner. This disease can be confirmed by the polymerase chain reaction technique and by measurement of 18-hydroxycortisol and 18-oxicortisol in a 24-hour urine sample. The disease is treated by the administration of glucocorticoid, which suppresses ACTH and thus expression of the hybrid gene. |
 |
| An important feature in the steroidogenic capacity of the zona glomerulosa is that it does not express CYP17. Therefore, zona glomerulosa cells never make cortisol, nor do they make adrenal androgens in any form. Pregnenolone is converted to progesterone and DOC by 3β-HSD and CYP21, respectively (Fig. 42-16).
|
| page 753 |  | | page 754 |
| Figure 42-16 Steroidogenic pathways in the zona glomerulosa. The first common reaction in the pathway, conversion of cholesterol to pregnenolone by CYP11A1, is not shown. Note that the last three reactions are catalyzed by CYP11B2. (Modified from Porterfield SP, White BA: Endocrine Physiology, 3rd ed. Philadelphia, Mosby, 2007.) |
| page 754 |  | | page 755 |
| Figure 42-17 The mineralocorticoid receptor (MR) is protected from activation by cortisol by the enzyme 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2), which converts cortisol to inactive cortisone. Cortisone can be converted back to cortisol in glucocorticoid target cells by the enzyme 11β-HSD type 1. GTF, general transcription factors; MRE, mineralocorticoid response element; GRE, glucocorticoid response element. (Modified from Porterfield SP, White BA: Endocrine Physiology, 3rd ed. Philadelphia, Mosby, 2007.) |
| A completely unique feature of the zona glomerulosa among the steroidogenic glands is its expression of CYP11B2, which is regulated by different signaling pathways. Furthermore, the enzyme coded by CYP11B2, called aldosterone synthase, catalyzes the last three reactions from DOC to aldosterone within the zona glomerulosa. These reactions are 11-hydroxylation of
DOC to form corticosterone, 18-hydroxylation to form 18-hydroxycorticosterone, and 18-oxidation to form aldosterone (Figs. 42-9 and 42-16).
|
| Transport and Metabolism of Aldosterone
|
| Aldosterone binds to albumin and corticosteroid-binding protein in blood with low affinity and therefore has a biological half-life of about 20 minutes. Almost all aldosterone is inactivated by the liver in one pass, conjugated to a glucuronide group, and excreted by the kidney.
|
| Mechanism of Aldosterone Action
|
| Clinical studies in humans have revealed a deleterious effect of aldosterone on cardiovascular function independent of its effects on renal sodium and water reabsorption. Aldosterone has a proinflammatory, profibrotic effect on the cardiovascular system and causes left ventricular hypertrophy and remodeling. This effect of aldosterone is associated with increased morbidity and mortality in patients with essential hypertension. |
 |
| Aldosterone acts much like cortisol (and other steroid hormones) in that its primary mechanism of action is mediated by binding to a specific intracellular receptor (i.e., mineralocorticoid receptor [MR]). After dissociation of chaperone proteins, nuclear translocation, dimerization, and binding to the mineralocorticoid response element (MRE), the aldosterone-MR complex regulates the expression of specific genes (see Chapter 3). Cortisol binds to the MR and activates the same genes as aldosterone does. However, as discussed earlier, some cells that express MR also express
11β-HSD2, which converts cortisol to the inactive steroid cortisone (Fig. 42-17). Cortisone can be converted back to cortisol by 11β-HSD1, which is expressed in several glucocorticoid-responsive tissues, including the liver and skin.
|
| Physiological Actions of Aldosterone
|
| The actions and regulation of aldosterone are discussed in Chapter 34.
|
| page 755 |  | | page 756 |
| Addison's disease is primary adrenal insufficiency, with both mineralocorticoids and glucocorticoids usually being deficient. In North America and Europe, the most prevalent cause of Addison's disease is autoimmune destruction of the adrenal cortex. Because of the cortisol deficiency, ACTH secretion increases. Elevated levels of ACTH can compete for MC1R in melanocytes and cause an increase in skin pigmentation, particularly in skin creases, scars, and gums (see Fig. 40-14). The loss of mineralocorticoids results in contraction of extracellular volume, which produces circulatory hypovolemia and therefore a drop in blood pressure. Because the loss of cortisol decreases the vasopressive response to catecholamines, peripheral vascular resistance drops, thereby facilitating the development of hypotension. Individuals with Addison's disease are also prone to hypoglycemia when stressed or fasting, and water intoxication can develop if excess water is ingested. Because cortisol is important for muscle function, muscle weakness also occurs in cortisol deficiency. The loss of cortisol results in anemia, decreased GI motility and secretion, and reduced iron and vitamin B12 absorption. Appetite decreases with cortisol deficiency, and this decreased appetite coupled with the GI dysfunction predisposes these individuals to weight loss. These patients often have disturbances in mood and behavior and are more susceptible to depression. |
| Adrenocortical hormone excess is termed Cushing's syndrome. Pharmacological use of exogenous corticosteroids is now the most common cause of Cushing's syndrome. The next most prevalent cause is ACTH-secreting tumors. The form of Cushing's syndrome caused by a functional pituitary adenoma is called Cushing's disease. The fourth most common cause of Cushing's syndrome is primary hypercortisolism resulting from a functional adrenal tumor. If the disorder is primary or if it is a result of corticosteroid treatment, secretion of ACTH will be suppressed and increased skin pigmentation will not occur. However, if hypersecretion of the adrenal is the result of an ACTH-secreting nonpituitary tumor, ACTH levels sometimes become high enough to increase skin pigmentation. |
| Increased cortisol secretion causes weight gain with a characteristic centripetal fat distribution and a "buffalo hump." The face will appear round (fat deposition), and the cheeks may be reddened, in part because of the polycythemia. The limbs will be thin as a result of skeletal muscle wasting (from increased proteolysis), and muscle weakness will be evident (from muscle proteolysis and hypokalemia). Proximal muscle weakness is apparent, so the patient may have difficulty climbing stairs or rising from a sitting position. The abdominal fat accumulation, coupled with atrophy of the abdominal muscles and thinning of the skin, will produce a large, protruding abdomen. Purple abdominal striae are seen as a result of damage to the skin by the prolonged proteolysis, increased intraabdominal fat, and loss of abdominal muscle tone. Capillary fragility occurs because of damage to the connective tissue supporting the capillaries. Patients are likely to show signs of osteoporosis and poor wound healing. They have metabolic disturbances that include glucose intolerance, hyperglycemia, and insulin resistance (Fig. 42-11). Prolonged hypercortisolism can lead to manifestations of diabetes mellitus. Because of suppression of the immune system caused by glucocorticoids, patients are more susceptible to infection. The mineralocorticoid activities of glucocorticoids and the possible increase in aldosterone secretion produce salt retention and subsequent water retention that result in hypertension. Excessive androgen secretion in women can produce hirsutism, male pattern baldness, and clitoral enlargement (adrenogenital syndrome). |
 |
| Any enzyme blockage that decreases cortisol synthesis will increase ACTH secretion and produce adrenal hyperplasia. The most common form of congenital adrenal hyperplasia occurs as a result of deficiency of the enzyme 21-hydroxylase (CYP21). These individuals cannot produce normal quantities of cortisol, deoxycortisol, DOC, corticosterone, or aldosterone (Figs. 42-8 and 42-10, C). Because of impaired cortisol production and resultant elevated ACTH levels, steroidogenesis is stimulated, thereby increasing the synthesis products "upstream" of the missing enzyme, as well as products of the zona reticularis. Because the latter include the adrenal androgens, a female fetus will be masculinized. Because they are unable to produce the mineralocorticoids, aldosterone, DOC, and corticosterone, patients with this disorder have difficulty retaining salt and maintaining extracellular volume. Consequently, they are likely to be hypotensive. If the blockage is at the next step, 11β-hydroxylase (CYP11B1), DOC will be formed and levels of DOC will accumulate (Figs. 42-8 and 42-10, C). Because DOC has significant mineralocorticoid activity and its levels become high, these individuals tend to retain salt and water and become hypertensive. |
 |
| page 756 |  | | page 757 |
- The adrenal gland is composed of a cortex that is of mesodermal origin and a medulla that is of neuroectodermal origin. The cortex produces steroid hormones, and the medulla produces catecholamines.
- The rate-limiting enzymes in medullary catecholamine synthesis are tyrosine hydroxylase and dopamine β-hydroxylase, which are induced by sympathetic stimulation, and phenylethanolamine-N-methyltransferase, which is induced by cortisol.
- Catecholamines increase serum glucose and fatty acid levels. They stimulate gluconeogenesis, glycogenolysis, and lipolysis. Catecholamines increase cardiac output but have selective effects on blood flow to different organs.
- Pheochromocytoma is a tumor of chromaffin tissue that produces excessive quantities of catecholamines. Symptoms of pheochromocytoma are often sporadic and include hypertension, headaches, sweating, anxiety, palpitations, chest pain, and orthostatic hypotension.
- The adrenal cortex displays clear structural and functional zonation: the zona glomerulosa produces the mineralocorticoid aldosterone, the zona fasciculata produces the glucocorticoid cortisol, and the zona reticularis produces the weak androgens DHEA and DHEAS.
- Cortisol binds to the glucocorticoid receptor. During stress, cortisol increases blood glucose by increasing gluconeogenesis in the liver and breaking muscle protein down to supply gluconeogenic precursors. Cortisol also decreases glucose uptake by muscle and adipose tissue and has permissive actions on glucagon and catecholamines. Cortisol has multiple effects on other tissue. From a pharmacological point of view, the most important is the immunosuppressive/antiinflammatory effect.
- Cortisol is regulated by the CRH-ACTH-cortisol axis. Cortisol negatively feeds back at the hypothalamus on both CRH-producing neurons and pituitary corticotropes. CRH is regulated by several forms of stress, including proinflammatory cytokines, hypoglycemia, neurogenic stress, and hemorrhage, and by diurnal input.
- The adrenal androgens DHEA, DHEAS, and androstenedione are androgen precursors. They can be converted to active androgens peripherally and provide about 50% of circulating androgens in women. In men, the role of adrenal androgens, if any, remains obscure. In women, adrenal androgens promote pubic and axillary hair growth and libido. Excessive adrenal androgens in women can lead to various degrees of virilization and ovarian dysfunction.
- The zona glomerulosa of the adrenal cortex is the site of aldosterone production. Aldosterone is the strongest naturally occurring mineralocorticoid in humans. It promotes Na+ and water reabsorption by the distal tubule and collecting duct while promoting renal K+ and H+ secretion. Aldosterone promotes Na+ and water absorption in the colon and salivary glands. It also has a proinflammatory, profibrotic effect on the cardiovascular system and causes left ventricular hypertrophy and remodeling.
- Major actions of angiotensin II on the adrenal cortex are increased growth and vascularity of the zona glomerulosa, increased StAR and CYP11B2 enzyme activity, and increased aldosterone synthesis.
- Major stimuli for aldosterone production are a rise in angiotensin II and a rise in serum [K+]. The major inhibitory signal is ANP.
- Addison's disease is adrenocortical insufficiency. Common symptoms include hypotension, hyperpigmentation, muscle weakness, anorexia, hypoglycemia, and hyperkalemic acidosis.
- Cushing's syndrome results from hypercortisolemia. If the basis of the disorder is increased pituitary adrenocorticotropin secretion, the disorder is called Cushing's disease. Common symptoms of Cushing's syndrome include centripetal fat distribution, muscle wasting, proximal muscle weakness, thin skin with abdominal striae, capillary fragility, insulin resistance, and polycythemia.
- Congenital adrenal hyperplasia is caused by a congenital enzyme deficiency that blocks the production of cortisol. The enzyme blockage results in elevated ACTH secretion, which stimulates adrenal cortical growth and secretion of precursors produced before the block. 21-Hydroxylase (CYP21B) deficiency is the most common form.
|
 |
|