| page 231 |  | | page 232 |
| page 232 |  | | page 233 |
| 12 Skeletal Muscle Physiology
|
| Muscle cells are highly specialized cells for the conversion of chemical energy to mechanical energy. Specifically, muscle cells use the energy in ATP to generate force or do work. Because work can take many forms (such as locomotion, pumping blood, or peristalsis), several types of muscle have evolved. The three basic types of muscle are skeletal muscle, cardiac muscle, and smooth muscle.
|
| Skeletal muscle acts on the skeleton. In limbs, for example, skeletal muscle spans a joint, thereby allowing a lever action. Skeletal muscle is under voluntary control (i.e., controlled by the central nervous system) and plays a key role in numerous activities such as maintenance of posture, locomotion, speech, and respiration. When viewed under the microscope, skeletal muscle exhibits transverse striations (at intervals of 2 to 3 μm) that result from the highly organized arrangement of actin and myosin molecules within the skeletal muscle cells. Thus, skeletal muscle is classified as a striated muscle. The heart is composed of cardiac muscle, and although it is also a striated muscle, it is an involuntary muscle (i.e., controlled by an intrinsic pacemaker and modulated by the autonomic nervous system). Smooth muscle (which lacks the striations evident in skeletal and cardiac muscle) is an involuntary muscle typically found lining hollow organs such as the intestine and blood vessels. In all three muscle types, force is generated by the interaction of actin and myosin molecules, a process that requires transient elevation of intracellular [Ca++].
|
| In this chapter attention is directed at the molecular mechanisms underlying contraction of skeletal muscle. Mechanisms for regulating the force of contraction are also addressed. To put this information into perspective, it is important to first examine the basic organization of skeletal muscle.
|
| ORGANIZATION OF SKELETAL MUSCLE
|
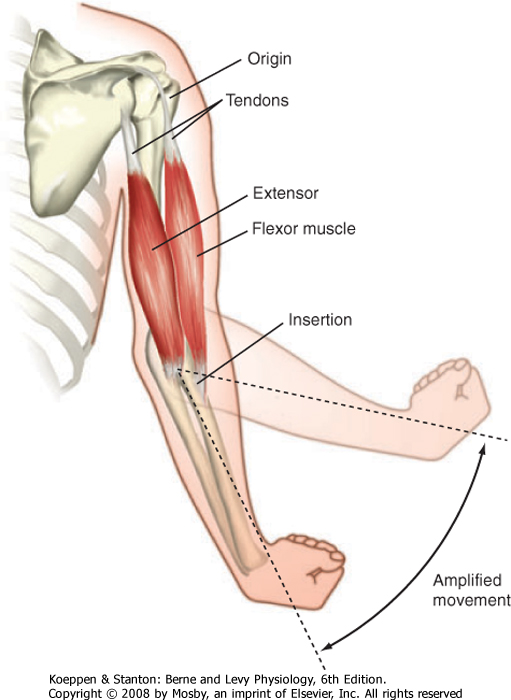
| Figure 12-1 illustrates skeletal muscles spanning the elbow joint. The muscles are attached to bone on either side of the joint. The point of attachment closest to the spine is called the origin, whereas the point of attachment on the distal region (on the far side of the joint) is called the insertion. These points of attachment occur through tendons (connective tissue) at the end of the muscle. Note that the point of insertion is close to the elbow joint, which promotes a broad range of motion. Also note that the joint is spanned by a flexor muscle on one side and an extensor muscle on the opposite side of the joint. Thus, contraction of the flexor muscle (see the biceps muscle in Fig. 12-1) results in a decrease in the angle of the elbow joint (bringing the forearm closer to the shoulder), whereas contraction of the extensor muscle (see the triceps muscle in Fig. 12-1) results in the reverse motion (extending the arm).
|
| The basic structure of skeletal muscle is shown in Figure 12-2. Each muscle is composed of numerous cells called muscle fibers. A connective tissue layer called the endomysium surrounds each of these fibers. Individual muscle fibers are then grouped together into fascicles, which are surrounded by another connective tissue layer called the perimysium. Within the perimysium are the blood vessels and nerves that supply the individual muscle fibers. Finally, fascicles are joined together to form the muscle. The connective tissue sheath that surrounds the muscle is called the epimysium. At the ends of the muscle, the connective tissue layers come together to form a tendon, which attaches the muscle to the skeleton. The connective tissue layers are composed mainly of elastin and collagen fibers, and they serve to transmit movement of the actin and myosin molecules to the skeleton to effect movement. The connective tissue layers also contribute to passive tension of muscle and prevent damage to the muscle fibers as a result of overstretching or contraction (or both).
|
| Individual skeletal muscle cells are narrow (≈10 to 80 μm in diameter), but they can be extremely long (up to 25 cm in length). Each skeletal muscle fiber contains bundles of filaments, called myofibrils, running along the axis of the cell. The gross striation pattern of the cell results from a repeating pattern in the myofibrils. Specifically, it is the regular arrangement of the thick and thin filaments within these myofibrils coupled with the highly organized alignment of adjacent myofibrils that gives rise to the striated appearance of skeletal muscle. Striations can be observed in intact muscle fibers and in the underlying myofibrils.
|
| page 233 |  | | page 234 |
| Figure 12-1 Skeletal muscle attaches to the skeleton by way of tendons and typically spans a joint. The proximal and distal points of attachment of the tendon are termed "origin" and "insertion," respectively. Note that the insertion is close to the joint, which allows a broad range of motion. Also note that skeletal muscles span both sides of the joint, which allows both flexion and extension of the forearm. |
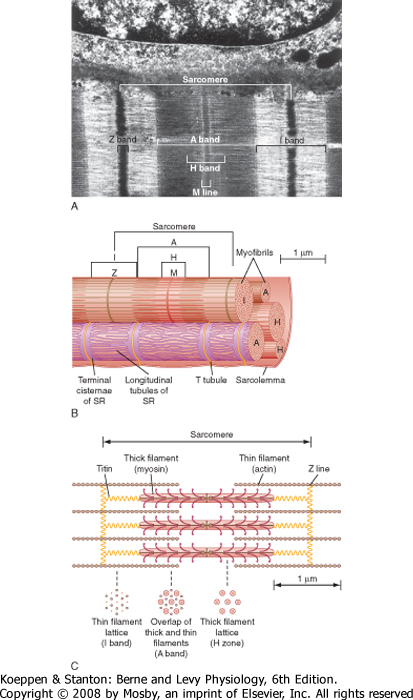
| A myofibril can be subdivided longitudinally into sarcomeres (Fig. 12-3). The sarcomere is demarcated by two dark lines called Z lines and represents a repeating contractile unit in skeletal muscle. The average length of a sarcomere is 2 μm. On either side
of the Z line is a light band (I band) that contains thin filaments composed primarily of the protein actin. The area between two I bands within a sarcomere is the A band, which contains thick filaments composed primarily of the protein myosin. The thin actin filaments extend from the Z line toward the center of the sarcomere and overlap a portion of the thick filaments. The dark area at the end of the A band represents this region of overlap between thick and thin filaments. A light area present in the center of the sarcomere is called the H band. This area represents the portion of the A band that contains myosin thick filaments, but no thin actin filaments. Thus, thin actin filaments extend from the Z line to the edge of the H band and overlap a portion of the thick filament in the A band. A dark line called the M line is evident in the center of the sarcomere and includes proteins that appear to be critical for organization and alignment of the thick filaments in the sarcomere.
|

|
| Figure 12-2 Skeletal muscle is composed of bundles of muscle fibers called a fasciculus. A muscle fiber represents an individual muscle cell and contains bundles of myofibrils. The striations are due to the arrangement of thick and thin filaments. See text for details. (Redrawn from Bloom W, Fawcett DW: A Textbook of Histology, 10th ed. Philadelphia, Saunders, 1975.) |
| As illustrated in Figure 12-3, each myofibril in a muscle fiber is surrounded by sarcoplasmic reticulum
(SR). The SR is an intracellular membrane network that plays a critical role in the regulation of intracellular [Ca++]. Invaginations of the sarcolemma, called T tubules, pass into the muscle fiber near the ends of the A band (i.e., close to the SR). The SR and the T tubules, however, are distinct membrane systems. The SR is an intracellular network, whereas the T tubules are in contact with the extracellular space. A gap (≈15 nm in width) separates the T tubules from the SR. The portion of the SR nearest the T tubules is called the terminal cisternae, and it is the site of Ca++ release, which is critical for contraction of skeletal muscle (see later). The longitudinal portions of the SR are continuous with the terminal cisternae and extend along the length of the sarcomere. This portion of the SR contains a high density of Ca++ pump protein (i.e., Ca++-ATPase), which is critical for reaccumulation of Ca++ in the SR and hence relaxation of the muscle.
|
| The thick and thin filaments are highly organized in the sarcomere of myofibrils (Fig. 12-3). As mentioned, thin actin filaments extend from the Z line toward the center of the sarcomere, whereas thick myosin filaments are centrally located and overlap a portion of the opposing thin actin filaments. The thick and thin filaments are oriented such that in the region of overlap within the sarcomere, each thick myosin filament is surrounded by a hexagonal array of thin actin filaments. It is the Ca++-dependent interaction of the thick myosin and the thin actin filaments that generates the force of contraction after stimulation of the muscle (see later).
|
| page 234 |  | | page 235 |
| Figure 12-3 A, Myofibrils are arranged in parallel within a muscle fiber. B, Each fibril is surrounded by sarcoplasmic reticulum (SR). Terminal cisternae of the SR are closely associated with T tubules and form a triad at the junction of the I and A bands. The Z lines define the boundary of the sarcomere. The striations are formed by overlap of the contractile proteins. Three bands can be seen, the A band, I band, and H band. An M line is seen in the middle of the H band. C, Organization of the proteins within a single sarcomere. The cross-sectional arrangement of the proteins is also illustrated. |
| The thick myosin filaments are tethered to the Z lines by a cytoskeletal protein called titin. Titin is a
very large elastic protein (molecular weight in excess of 3000 kDa) that extends from the Z line to the center of the sarcomere and appears to be important for organization and alignment of the thick filaments in the sarcomere. Titin may also serve as a mechanosensor and influence gene expression and protein degradation in a mechanical activity-dependent manner. Some forms of muscular dystrophy have been attributed to defects in titin.
|
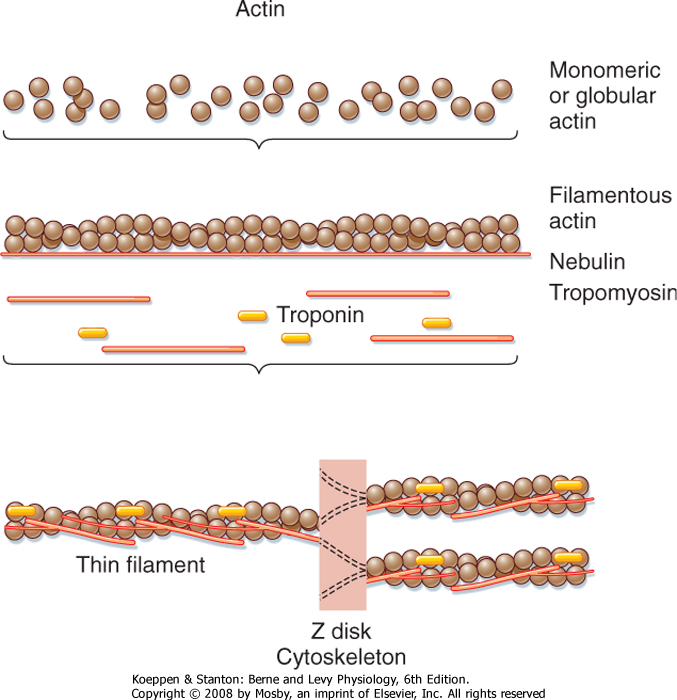
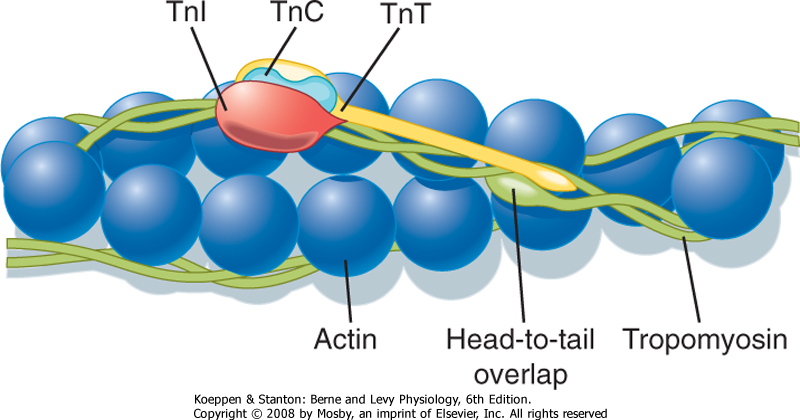
| The thin filament is formed by the aggregation of actin molecules (termed globular actin or G-actin) into a two-stranded helical filament called F-actin, or filamentous actin (Fig. 12-5). The elongated cytoskeletal protein nebulin extends along the length of the thin filament and may participate in regulation of the length of the thin filament. Dimers of the protein tropomyosin extend over the entire actin filament and cover myosin binding sites on the actin molecules. Each tropomyosin dimer extends over seven actin molecules, with sequential tropomyosin dimers arranged in a head-to-tail configuration. A troponin complex consisting of three subunits (troponin T, troponin I, and troponin C) is present on each tropomyosin dimer and influences the position of the tropomyosin molecule on the actin filament and hence the ability of tropomyosin to inhibit binding of myosin to the actin filament. Troponin T binds tropomyosin, troponin I facilitates the inhibition of myosin binding to actin by tropomyosin, and troponin C binds Ca++. Binding of Ca++ to troponin C promotes the movement of tropomyosin on the actin filament, thereby exposing myosin binding sites and facilitating the interaction of myosin and actin filaments and sarcomere contraction (see later). Additional proteins associated with the thin filament include tropomodulin, α-actinin, and capZ protein. Tropomodulin is located at the end of the thin filament, toward the center of the sarcomere, and may participate in setting the length of the thin filament. α-Actinin and capZ protein serve to anchor the thin filament to the Z line.
|
| page 235 |  | | page 236 |
| The muscular dystrophies constitute a group of genetically determined degenerative disorders. Duchenne's muscular dystrophy (DMD; described by G.B. Duchenne in 1861) is the most common of the muscular dystrophies and affects 1 in 3500 boys (3 to 5 years of age). Severe muscle wasting occurs, with most patients being wheelchair bound by the age of 12 and many dying of respiratory failure in adulthood (30 to 40 years of age). DMD is an X-linked recessive disease that has been linked to a defect in the dystrophin gene that leads to a deficiency of the dystrophin protein in skeletal muscle, brain, retina, and smooth muscle. Dystrophin is a large (427 kDa) protein that is present in low abundance (0.025%) in skeletal muscle. It is localized on the intracellular surface of the sarcolemma in association with several integral membrane glycoproteins (forming a dystrophin-glycoprotein complex). This dystrophin-glycoprotein complex provides a structural link between the subsarcolemmal cytoskeleton of the muscle cell and the extracellular matrix (Fig. 12-4) and appears to stabilize the sarcolemma and hence prevents contraction-induced injury (rupture). The dystrophin-glycoprotein complex may also serve as a scaffold for cell signaling cascades that promote cell survival. |
| Although defects in the dystrophin-glycoprotein complex are involved in many forms of muscular dystrophy, recent studies have identified some forms of muscular dystrophy that involve other mechanisms. Specifically, a defect in sarcolemma repair (attributed to loss/mutation of the protein dysferlin) appears to underlie at least one form of muscular dystrophy (limb-girdle muscular dystrophy 2B, associated with muscle wasting in the pelvic region). Defects in the protein titin (termed titinopathies) have been implicated in other forms of muscular dystrophy (e.g., limb-girdle muscular dystrophy 2J and tibial muscular dystrophy). The link between titin mutations and muscular dystrophy may reflect a disruption in the ability of titin to bind a signalosome that can inhibit transcription and promote protein degradation. In the latter mechanism, the signalosome has been shown to include a muscle-specific ubiquitin ligase (viz., MuRF2) that can inhibit a transcription factor (viz., serum response factor) by promoting translocation to the cytosol and promote protein degradation (through ubiquitination-see Chapter 1). Mutations in the protease calpain 3 (resulting in loss of protease activity) have also been implicated in some types of muscular dystrophy (e.g., limb-girdle muscular dystrophy 2A), apparently secondary to apoptosis. |
 |

|
| Figure 12-4 Organization of the dystrophin-glycoprotein complex in skeletal muscle. The dystrophin-glycoprotein complex provides a structural link between the cytoskeleton of the muscle cell and the extracellular matrix, which appears to stabilize the sarcolemma and hence prevents contraction-induced injury (rupture). Duchenne's muscular dystrophy is associated with loss of dystrophin. |
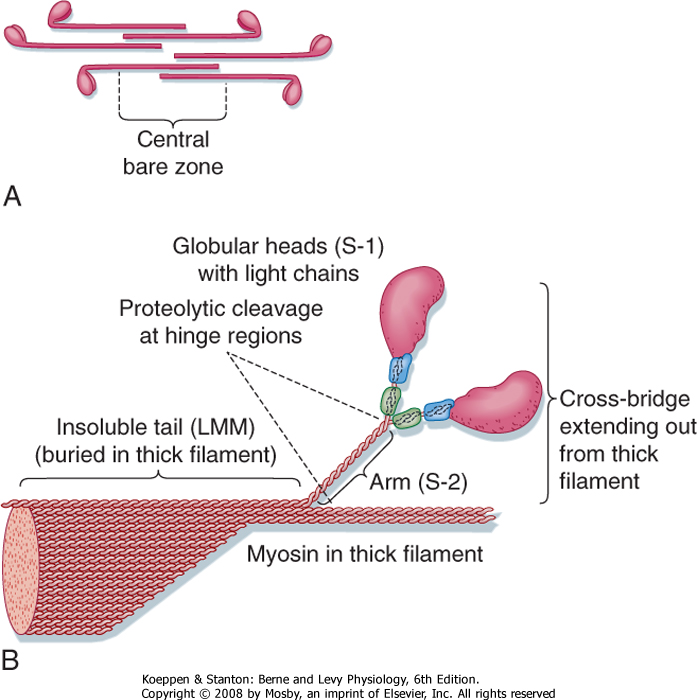
| Organization of the thick filament is shown in Figure 12-6. Myosin is a large protein (≈480 kDa) that consists of six different polypeptides with one pair of large heavy chains (≈200 kDa) and two pairs of light chains (≈20 kDa). The heavy chains are wound together in an α-helical configuration to form a long rod-like segment, with the N-terminal portion of each heavy chain forming a large globular head. The head region extends away from the thick filament toward the actin thin filament
and is the portion of the molecule that can bind to actin. Myosin is also able to hydrolyze ATP, and ATPase activity is located in the globular head as well. The two pairs of light chains are associated with the globular head. One of these pairs of light chains, termed essential light chains, is critical for the ATPase activity of myosin. The other pair of light chains, sometimes called regulatory light chains, may influence the kinetics of myosin and actin binding under certain conditions. Thus, myosin ATPase activity resides in the globular head of myosin and requires the presence of light chains (viz., the "essential" light chains).
|
| page 236 |  | | page 237 |
| Figure 12-5 Organization of a thin filament. Polymerization of monomeric actin into filamentous actin forms the backbone of the thin filament. The filament contains several other structural/regulatory proteins such as nebulin, tropomyosin, and troponin. |
| Myosin filaments form by a tail-to-tail association of myosin molecules, thereby resulting in a bipolar arrangement of the thick filament. The thick filament then extends on either side of the central bare zone by a head-to-tail association of myosin molecules, thus maintaining the bipolar organization of the thick filament centered on the M line. Such a bipolar arrangement
is critical for drawing the Z lines together (i.e., shortening the length of the sarcomere) during contraction. The mechanisms controlling this highly organized structure of the myosin thick filament are not clear, although the cytoskeletal protein titin is thought to participate in the formation of a scaffold for organization and alignment of the thick filament in the sarcomere. Additional proteins found in the thick filaments (e.g., myomesin and C protein) may also participate in the bipolar organization or packing of the thick filament (or both).
|
| CONTROL OF SKELETAL MUSCLE ACTIVITY
|
| Motor Nerves and Motor Units
|
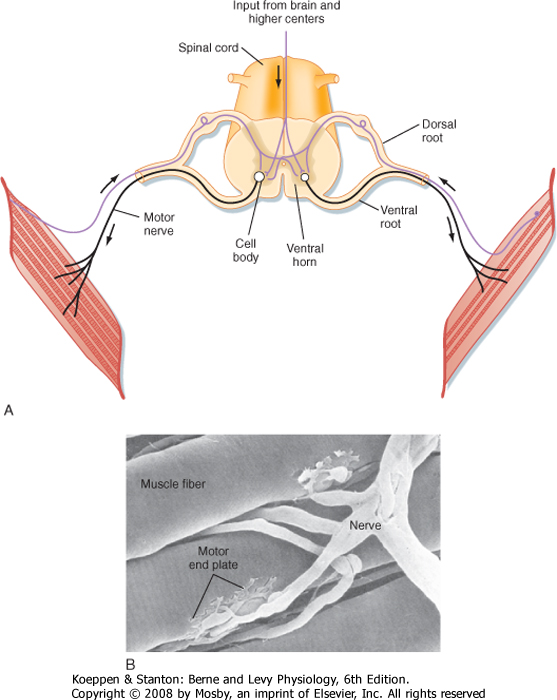
| Skeletal muscle is controlled by the central nervous system. Specifically, each skeletal muscle is innervated by an α motor neuron. The cell bodies of α motor neurons are located in the ventral horn of the spinal cord (Fig. 12-7; see also Chapter 9). The motor axons exit via the ventral roots and reach the muscle through mixed peripheral nerves. The motor nerves branch in the muscle, with each branch innervating a single muscle fiber. The specialized cholinergic synapse that forms the neuromuscular junction and the neuro-muscular transmission process that generates an action potential in the muscle fiber are described in Chapter 6.
|
|
| Figure 12-6 Organization of a thick filament. A thick filament is formed by the polymerization of myosin molecules in a tail-to-tail configuration extending from the center of the sarcomere (A). An individual myosin molecule has a tail region and a cross-bridge region. The cross-bridge region is composed of an arm and globular heads (B). The globular heads contain light chains that are important for the function of myosin ATPase activity. |
| A motor unit consists of the motor nerve and all the muscle fibers innervated by the nerve. The motor unit is the functional contractile unit because all the muscle cells within a motor unit contract synchronously when the motor nerve fires. The size of motor
units within a muscle varies depending on the function of the muscle. In the rectus muscles of the eye the motor units are small (i.e., only a small number of muscle fibers are innervated by a motor neuron), and thus movement of the eye can be precisely controlled. In contrast, the motor units of the legs are large, which facilitates running. Activation of varying numbers of motor units within a muscle is one way in which the tension developed by a muscle can be controlled (see later).
|
| The neuromuscular junction formed by the α motor neuron is called an end plate (see Chapter 6 for details). Acetylcholine released from the α motor neuron at the neuromuscular junction initiates an action potential in the muscle fiber that rapidly spreads along its length. The duration of the action potential in skeletal muscle is less than 5 msec. This contrasts with the duration of the action potential in cardiac muscle, which is approximately 200 msec. The short duration of the skeletal muscle action potential allows very rapid contractions of the fiber and provides yet another mechanism by which the force of contraction can be increased. Increasing tension by repetitive stimulation of the muscle is called tetany (this phenomenon is described in more detail later in this chapter).
|
| Excitation-Contraction Coupling
|
| page 237 |  | | page 238 |
| Figure 12-7 Skeletal muscle is a voluntary muscle controlled by the central nervous system, with efferent signals (i.e., action potentials) passing through an α motor neuron to muscle fibers. Each motor neuron may innervate many muscle fibers within a muscle, although each muscle fiber is innervated by only one motor neuron (A). B, Scanning electron micrograph showing innervation of several muscle fibers by a single motor neuron. (B, From Bloom W, Fawcett DW: A Textbook of Physiology, 12th ed. New York, Chapman & Hall, 1994.) |
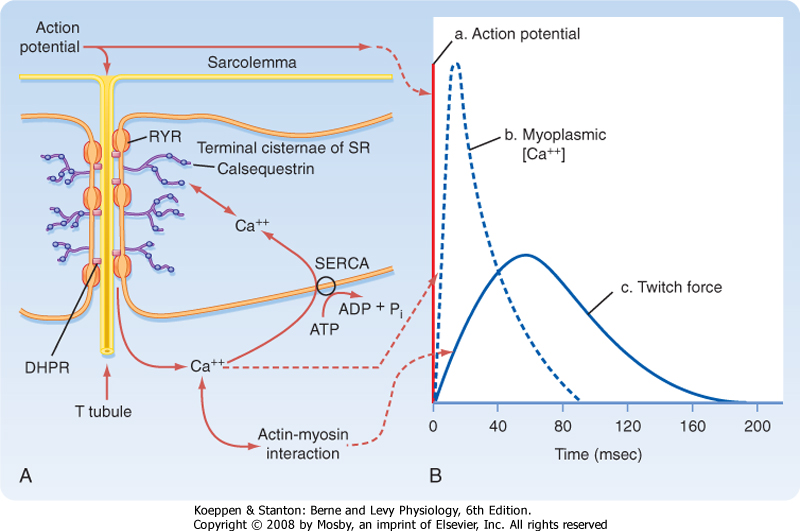
| When an action potential is transmitted along the sarcolemma of the muscle fiber and then down the T tubules, Ca++ is released from the terminal cisternae
SR into the myoplasm. This release of Ca++ from the SR raises intracellular [Ca++], which in turn promotes actin-myosin interaction and contraction. The time course for the increase in intracellular [Ca++] relative to the action potential and development of force is shown in Figure 12-8. The action potential is extremely short-lived (≈5 msec). The elevation in intracellular [Ca++] begins slightly after the action potential and peaks at approximately 20 msec. This increase in intracellular [Ca++] initiates a contraction called a twitch.
|
| The mechanism underlying the elevation in intracellular [Ca++] involves an interaction between protein in the T tubule and the adjacent terminal cisternae of the SR. As previously described (Fig. 12-3), the T tubule represents an invagination of the sarcolemma that extends into the muscle fiber and forms a close association with two terminal cisternae of the SR. The association of a T tubule with two opposing terminal cisternae is called a triad. Although there is a gap (≈15 nm in width) between the T tubule and the terminal cisternae, proteins bridge this gap. Based on their appearance on electron micrographs, these bridging proteins are called feet (Fig. 12-9). These feet are the Ca++ release channels in the membrane of the terminal cisternae that are responsible for the elevation in intracellular [Ca++] in response to the action potential. Because this channel binds the drug ryanodine, it is commonly called the ryanodine receptor (RYR). RYR is a large protein (≈500 kDa) that exists as a homotetramer. Only a small portion of the RYR molecule is actually embedded in the SR membrane. Most of the RYR molecule appears to be in the myoplasm and spans the gap between the terminal cisternae and the T tubule (Fig. 12-10).
|
| page 238 |  | | page 239 |
| Figure 12-8 Stimulation of a skeletal muscle fiber initiates an action potential in the muscle that travels down the T tubule and induces release of Ca++ from the terminal cisternae of the SR (A). The rise in intracellular [Ca++] causes a contraction. As Ca++ is pumped back into the SR by Ca++-ATPase (SERCA), relaxation occurs. B, Time courses of the action potential, myoplasmic Ca++ transient, and force of the twitch contraction. |
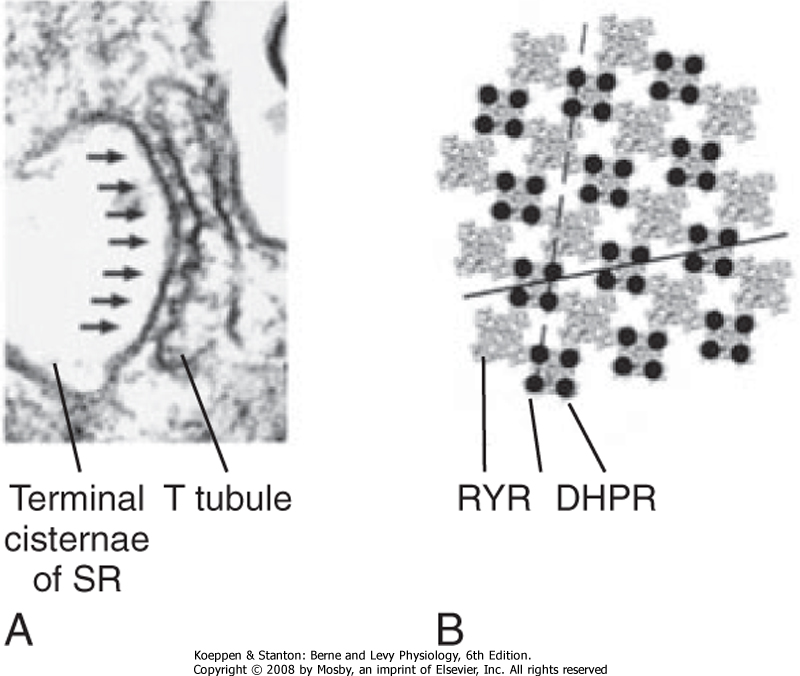
| Figure 12-9 A, Electron micrograph of a triad illustrating the "feet" between the T tubule and the SR, which are thought to be the ryanodine receptors (RYRs) in the SR. B, Each RYR in the SR is associated with four dihydropyridine receptors (DHPRs) in the T tubule. (From Protasi F et al: Biophys J 79:2494, 2000.) |
| At the T-tubule membrane, the RYR is thought to interact with a protein called the dihydropyridine receptor (DHPR). DHPR is an L-type voltage-gated Ca++ channel with five subunits. One of these subunits binds the dihydropyridine class of channel blocking drugs and appears to be critical for the ability of the action potential in the T tubule to induce release of Ca++ from the SR. However, influx of Ca++ into the cell through the DHPR is not needed for the initiation of Ca++ release from the SR. Indeed, skeletal muscle is able to contract in the absence of extracellular Ca++ or with a mutated DHPR that does not conduct Ca++. Instead, release of Ca++ from the terminal cisternae of the SR is thought to result from a conformational change in the DHPR as the action potential passes down the T tubule, and this conformational change in the DHPR, by means of a protein-protein interaction, opens the RYR and releases Ca++ into the myoplasm.
|
| Structural analysis, including the use of freeze-fracture techniques, provides evidence for a close physical association of DHPR and RYR (Fig. 12-9). DHPR in the T-tubule membrane appears to reside directly opposite the four corners of the underlying homotetrameric RYR channel in the SR membrane.
|
| page 239 |  | | page 240 |
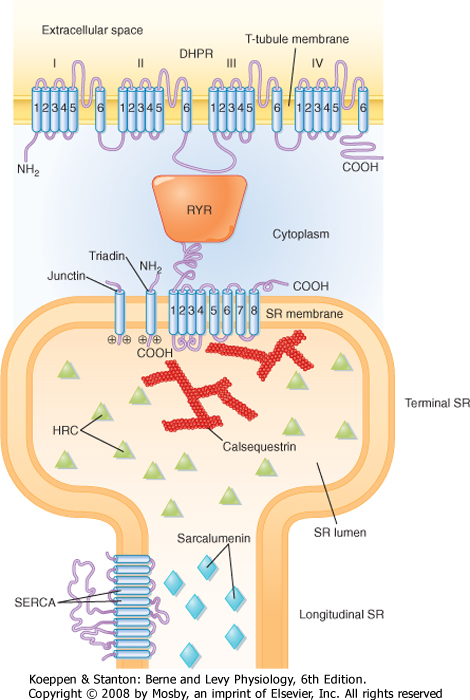
| Figure 12-10 Molecular structure and relationships between the dihydropyridine receptor (DHPR) in the T-tubule membrane and the RYR in the SR membrane. Triadin is an associated SR protein that may participate in the interaction of RYR and DHPR. Calsequestrin is a low-affinity Ca++-binding protein that helps accumulate Ca++ in the terminal cisternae. See text for details. (From Rossi AE, Dirksen RT: Muscle Nerve 33:715, 2006.) |
| A variety of mutational studies have been conducted to ascertain the region of the DHPR that is critical for opening of the RYR. One possible site of interaction (depicted in Fig. 12-10) is the myoplasmic loop between transmembrane domains II and III in the α1 subunit of the DHPR. The voltage-sensing region of the DHPR involved in intramembranous charge movement is thought to reside in the S4 transmembrane segments of the α1 subunit. Genetic mutations in the RYR or DHPR, or in both, have been associated with pathological disturbances in myoplasmic [Ca++]. Such disturbances include malignant hyperthermia and central core disease, as described later. These mutations are typically observed in the myoplasmic portion of the RYR, although mutations have also been observed in a myoplasmic loop in the DHPR. |
 |
| Other proteins that reside near the RYR include calsequestrin, triadin, and junctin (Fig. 12-10). Calsequestrin is a low-affinity Ca++-binding protein that is present in the lumen of the terminal cisternae. It allows Ca++ to be "stored" at high concentration and thereby establishes a favorable concentration gradient that facilitates the efflux of Ca++ from the SR into the myoplasm when the RYR opens. Triadin and junctin are in the terminal cisternae membrane and bind both RYR and calsequestrin; they could anchor calsequestrin near the RYR and thereby increase Ca++ buffering capacity at the site of Ca++ release. Histidine-rich calcium-binding protein (HRC) is another low-affinity Ca++-binding protein in the SR lumen, although it is less abundant than calsequestrin. HRC appears to bind triadin in a Ca++-dependent manner, which raises the
possibility of a role greater than serving simply as a Ca++ buffer.
|
| page 240 |  | | page 241 |
| Relaxation of skeletal muscle occurs as intracellular Ca++ is resequestered by the SR. Uptake of Ca++ into the SR is due to the action of a Ca++ pump (i.e., Ca++-ATPase). This pump is not unique to skeletal muscle and is found in all cells in association with the endoplasmic reticulum. Accordingly, it is named SERCA, which stands for sarcoplasmic endoplasmic reticulum calcium ATPase. SERCA is the most abundant protein in the SR of skeletal muscle, and it is distributed throughout the longitudinal tubules and the terminal cisternae as well. It transports two molecules of Ca++ into its lumen for each molecule of ATP hydrolyzed.* Thus, the Ca++ transient seen during a twitch contraction (see Fig. 12-8) reflects release of Ca++ from the
terminal cisternae via the RYR and reuptake primarily into the longitudinal portion of the SR by SERCA. The low-affinity Ca++-binding protein sarcalumenin is present throughout the longitudinal tubules of the SR and nonjunctional regions of the terminal cisternae and is thought to be involved in the transfer of Ca++ from sites of Ca++ uptake in the longitudinal tubules to sites of Ca++ release in the terminal cisternae. Recent studies suggest that sarcalumenin increases Ca++ uptake by SERCA, at least in part by buffering luminal Ca++ near the pump.
|
| Actin-Myosin Interaction: Cross-Bridge Formation
|
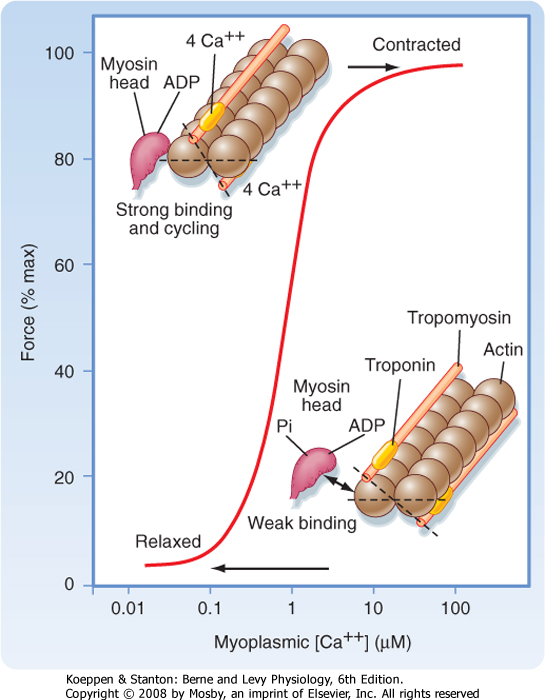
| Figure 12-11 The contractile force of skeletal muscle increases in a Ca++-dependent manner as a result of binding of Ca++ to troponin C and the subsequent movement of tropomyosin away from myosin binding sites on the underlying actin molecules. See text for details. (From MacLennan DH et al: J Biol Chem 272:28815, 1997.) |
| Genetic diseases causing disturbances in Ca++ homeostasis in skeletal muscle include malignant hyperthermia (MH), central core disease (CCD), and Brody's disease (BD). MH is an autosomal dominant trait that has life-threatening consequences in certain surgical instances. Anesthetics such as halothane or ether and the muscle relaxant succinylcholine can produce uncontrolled release of Ca++ from the SR, thereby resulting in skeletal muscle rigidity, tachycardia, hyperventilation, and hyperthermia. This condition is lethal if not treated immediately. There are currently a series of tests (using contractile responses of muscle biopsy specimens) to assess whether a patient has MH. The incidence of MH is approximately 1 in 15,000 children and 1 in 50,000 adults treated with anesthetics. MH is the result of a defect in the SR Ca++ release channel (RYR), which becomes activated in the presence of the aforementioned anesthetics and results in the release of Ca++ into the myoplasm and hence prolonged muscle contraction (rigidity). The defect in the RYR is not restricted to a single locus. In some cases MH has been linked to a defect in the DHPR of the T tubule. |
| CCD is a rare autosomal dominant trait that results in muscle weakness, loss of mitochondria in the core of skeletal muscle fibers, and some disintegration of contractile filaments. CCD is often closely associated with MH, so CCD patients are treated as though they are susceptible to MH in surgical situations. It is hypothesized that central cores devoid of mitochondria represent areas of elevated intracellular Ca++ secondary to a mutation in the RYR. The loss of mitochondria is thought to occur when they take up the elevated Ca++ leading to mitochondrial Ca++ overload. |
| BD is characterized by painless muscle cramping and impaired muscle relaxation during exercise. While running upstairs, for example, muscles may stiffen and temporarily cannot be used. This relaxation abnormality is seen in muscles of the legs, arms, and eyelid, with the response worsened in cold weather. BD can be either autosomal recessive or autosomal dominant and may involve mutations in up to three genes. BD, however, is a rare occurrence (affecting 1 in 10,000,000 births). It appears that BD results from decreased activity of the SERCA1 Ca++ pump found in fast-twitch skeletal muscle (see later). The decreased activity of SERCA1 has been associated with mutation in the SERCA1 gene, although there may also be an accessory factor that contributes to the decreased SR Ca++ uptake in the fast-twitch skeletal muscle of individuals with BD. |
| page 241 |  | | page 242 |
| Figure 12-12 Organization of the thin filament showing a double-helical array of tropomyosin on the actin filament, with sequential tropomyosin molecules arranged in a head-to-tail configuration. Such a configuration may promote the interaction of one tropomyosin unit with an adjacent tropomyosin. Also shown is the troponin complex consisting of its three subunits: troponin C (TnC), troponin I (TnI), and troponin T (TnT). See text for details. (From Gordon AM et al: Physiol Rev 80:853, 2000.) |
| As noted, contraction of skeletal muscle requires an increase in intracellular [Ca++]. Moreover, the process of contraction is regulated by the thin filament. As shown in Figure 12-11, contractile force (i.e., tension) increases in sigmoidal fashion as intracellular [Ca++] is elevated above 0.1 μm, with half-maximal force occurring at less than 1 μm Ca++. The mechanism by which Ca++ promotes this increase in tension is as follows. Ca++ released from the SR binds to troponin C. Once bound with Ca++, troponin C facilitates movement of the associated tropomyosin molecule toward the cleft of the actin filament. This movement of tropomyosin
exposes the myosin binding site on the actin filament and allows a cross-bridge to form and thereby generate tension (see later). Troponin C has four Ca++ binding sites. Two of these sites have high affinity for Ca++ but also bind Mg++ at rest. These sites seem to be involved in controlling and enhancing the interaction between the troponin I and troponin T subunits. The other two
binding sites have lower affinity and bind Ca++ as its concentration rises after release from the SR. Binding of myosin to the actin filaments appears to cause a further shift in tropomyosin. Although a given tropomyosin molecule extends over seven actin molecules, it is hypothesized that the strong binding of myosin to actin results in movement of an adjacent tropomyosin molecule, perhaps exposing myosin binding sites on as many as 14 actin molecules. This ability of one tropomyosin molecule to influence the movement of another may be a consequence of the close proximity of adjacent tropomyosin molecules (Fig. 12-12).
|
| Cross-Bridge Cycling- Sarcomere Shortening
|
| Figure 12-13 Cross-bridge cycle. State a, In the relaxed state, ATP is partially hydrolyzed (M · ADP · Pi). State b, In the presence of elevated myoplasmic Ca++, myosin binds to actin. State c, Hydrolysis of ATP is completed and causes a conformational change in the myosin molecule that pulls the actin filament toward the center of the sarcomere. State d, A new ATP binds to myosin and causes release of the cross-bridge. Partial hydrolysis of the newly bound ATP recocks the myosin head, which is now ready to bind again and again. If myoplasmic [Ca++] is still elevated, the cycle repeats. If myoplasmic [Ca++] is low, relaxation results. |
| Once myosin and actin have bound, ATP-dependent conformational changes in the myosin molecule result in movement of the actin filaments toward the center of the sarcomere. Such movement shortens the length of the sarcomere and thereby contracts the muscle fiber. The mechanism by which myosin produces force and shortens the sarcomere is thought to involve four basic steps that are collectively termed the cross-bridge cycle (labeled a to d in Figure 12-13). In the resting state, myosin is thought to have partially hydrolyzed ATP (state a). When Ca++ is released from the terminal cisternae of the SR, it binds to troponin C, which in turn promotes movement of tropomyosin on the actin filament such that myosin binding sites on actin are exposed. This then allows the "energized" myosin head to bind to the underlying actin (state b). Myosin next undergoes a conformational change termed "ratchet action" that pulls the actin filament toward the center of the sarcomere (state c). Myosin releases ADP and Pi during the transition to state c. Binding of ATP to myosin decreases the affinity of myosin for actin, thereby resulting in the release of myosin from the actin filament (state d). Myosin then partially hydrolyzes the ATP, and part of the energy in the ATP is used to recock the head and return to the resting state. If intracellular [Ca++] is still elevated,
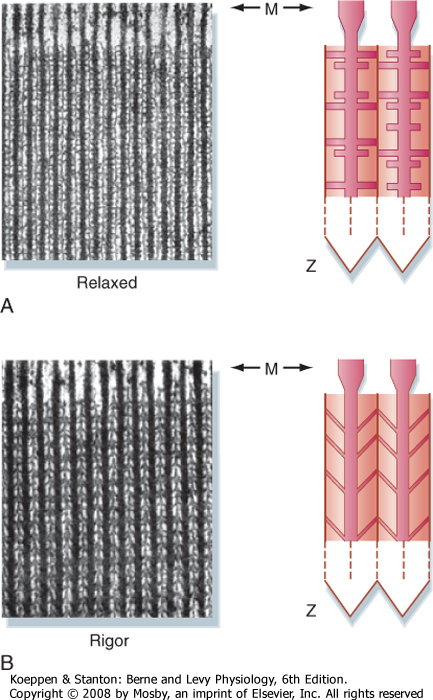
myosin will undergo another cross-bridge cycle and produce further contraction of the muscle. The ratchet action of the cross-bridge is capable of moving the thin filament approximately 10 nm. The cycle continues until the SERCA pumps Ca++ back into the SR. As [Ca++] falls, Ca++ dissociates from troponin C, and the troponin-tropomyosin complex moves and blocks the myosin binding sites on the actin filament. If the supply of ATP is exhausted, as occurs with death, the cycle stops in state "c" with the formation of permanent actin-myosin complexes (i.e., the rigor state). In this state the muscle is rigid and the condition is termed "rigor mortis."
|
| page 242 |  | | page 243 |
| Figure 12-14 Electron micrograph of skeletal muscle in the relaxed and contracted (rigor) states. The direction of the cross-bridges in the contracted state is consistent with a ratchet action of myosin, which pulls actin toward the center of the sarcomere. (Modified from Patton H et al: Textbook of Physiology. Philadelphia, Saunders, 1989.) |
| As already noted, formation of the thick filaments involves the association of myosin molecules in a tail-to-tail configuration to produce a bipolar orientation (Fig. 12-6). Such a bipolar orientation allows myosin to pull the actin filaments toward the center of the sarcomere during the cross-bridge cycle. The myosin molecules are also oriented in a helical array in the thick filament such that cross-bridges extend toward each of the six thin filaments surrounding the thick filament (Fig. 12-3). These myosin projections/cross-bridges can be seen on electron micrographs of skeletal muscle (Fig. 12-14) and appear to extend perpendicular from the thick filaments at rest. In the
contracted state, the myosin cross-bridges slant toward the center of the sarcomere, consistent with the ratchet action of the myosin head.
|
| The cross-bridge cycling mechanism just described is called the sliding filament theory because the myosin cross-bridge is pulling the actin thin filament toward the center of the sarcomere, thereby resulting in an apparent "sliding" of the thin filament past the thick filament. There is, however, uncertainty about how many myosin molecules contribute to the generation of force and whether both myosin heads in a given myosin molecule are involved. It has been calculated that there may be 600 myosin heads per thick filament, with a stoichiometry of 1 myosin head per 1.8 actin molecules. As a result of steric considerations, it is unlikely that all myosin heads can interact with actin, and calculations suggest that even during maximal force generation, only 20% to 40% of the myosin heads bind to actin.
|
|
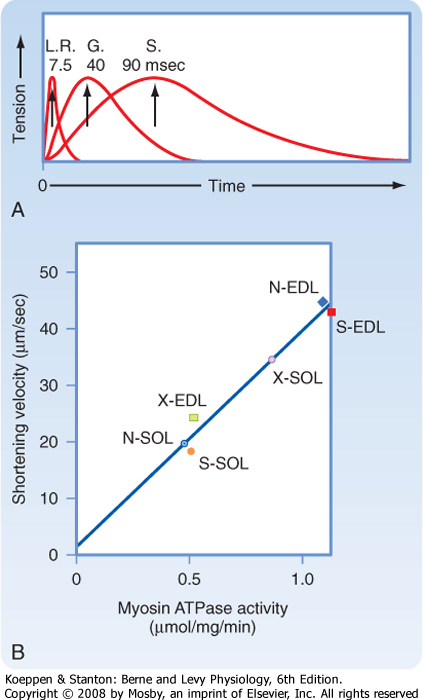
| Figure 12-15 A, Muscles vary in terms of the speed of contraction. G, gastrocnemius of the leg; LR, lateral rectus muscle of the eye; S, soleus muscle of the leg. B, The speed of shortening is correlated with myosin ATPase activity. (A, From Montcastle V [ed]: Medical Physiology, 12th ed. St. Louis, Mosby, 1974; B, from Barany M, Close RI: J Physiol 213:455, 1971.) N-SOL, normal soleus (slow twitch); N-EDL, normal extensor digitorum longus (fast twitch); S-EDL, self-innervated EDL (EDL motor nerve transected and resutured); S-SOL, self-innervated soleus (soleus motor nerve transected and resutured); X-EDL, cross innervated EDL (EDL innervated by soleus motor nerve); X-SOL, cross innervated SOL (soleus innervated by EDL motor nerve). |
| The conversion of chemical energy (i.e., ATP) to mechanical energy by muscle is highly efficient. In isolated muscle preparations, maximum mechanical efficiency (≈65% efficiency) is obtained at a submaximal force of 30% maximal tension. In humans performing
steady-state ergometer exercise, mechanical efficiencies range from 40% to 57%.
|
| Skeletal muscle can be classified as either fast-twitch (also called type IIA and type IIB) or slow-twitch (also called type I) muscle. As shown in Figure 12-15, the lateral rectus muscle of the eye contracts very quickly, with peak tension attained within 7.5 msec after stimulation. The gastrocnemius muscle of the leg, in contrast, requires 40 msec to develop peak tension. The soleus muscle of the leg requires even longer (≈90 msec) for peak tension to develop. Thus, the soleus muscle is classified as a slow-twitch muscle, whereas the lateral rectus muscle would be classified as a fast-twitch muscle. The gastrocnemius muscle contains a mixture of fast- and slow-twitch fibers and thus exhibits a weighted average intermediate rate of tension development when the whole muscle is stimulated.
|
| page 243 |  | | page 244 |
|
Table 12-1.
Basic Classification of Skeletal Muscle Fiber Types |
| | Type I: Slow Oxidative (Red) | Type IIB: Fast Glycolytic (White) | Type IIA*: Fast Oxidative (Red) |
| Myosin isoenzyme (ATPase rate) | Slow | Fast | Fast |
| Sarcoplasmic reticular Ca++ pumping capacity | Moderate | High | High |
| Diameter (diffusion distance) | Moderate | Large | Small |
| Oxidative capacity: mitochondrial content, capillary density, myoglobin | High | Low | Very high |
| Glycolytic capacity | Moderate | High | High |
*Comparatively infrequent in humans and other primates. In text, the simple designation of type II fiber refers to a fast glycolytic (type IIB) fiber.
|
| A correlation between speed of contraction and myosin ATPase activity is also seen and reflects the expression of different myosin isoforms in the two muscle fiber types (Fig. 12-15). Although the basic structure of the myosin isoforms in fast-twitch and slow-twitch muscles is similar (i.e., two heavy chains with two pairs of light chains), they are products of different genes and thus have different amino acid sequences.
|
| Fast and slow fibers can be distinguished not only on the basis of myosin ATPase activity but also by the activities of enzymes in the oxidative and glycolytic metabolic pathways (Table 12-1). In most fast fibers, the activity of glycolytic enzymes is high and the activity of oxidative enzymes is low. These characteristics correlate with the number of mitochondria present in the fiber. Electron micrographs of fast fibers show only a few mitochondria as compared with the large number seen in slow fibers. Fast fibers also have a much more extensive SR than slow fibers do. Typically, fast fibers and slow fibers are intermixed in most mammalian skeletal muscles.
|
| Because of the dependence of fast fibers on glycolytic metabolism, they fatigue rapidly. Consequently, they are used only occasionally and for brief periods. In contrast, slow fibers meet their metabolic demands by oxidative phosphorylation. As a result, these muscles fatigue more slowly and are therefore used for more sustained activities (e.g., maintenance of posture). Some fast fibers have both high glycolytic and high oxidative capacity. Such fibers, called type IIA, are found in mammals but are uncommon in humans. The fibers that derive their energy primarily from oxidative phosphorylation (i.e., the slow type I fibers and the fast type IIA fibers) contain numerous mitochondria and high levels of the oxygen-binding protein myoglobin. Because myoglobin is red, these fibers are sometimes called "red fibers." Table 12-2 summarizes some of the differences in the motor units of fast and slow muscles.
|
|
Table 12-2.
Properties of Motor Units |
| Characteristics | Motor Unit Classification |
| | Type I | Type II |
| Properties of Nerve |
| Cell diameter | Small | Large |
| Conduction velocity | Fast | Very fast |
| Excitability | High | Low |
| Properties of Muscle Cells |
| Number of fibers | Few | Many |
| Fiber diameter | Moderate | Large |
| Force of unit | Low | High |
| Metabolic profile | Oxidative | Glycolytic |
| Contraction velocity | Moderate | Fast |
| Fatigability | Low | High |
| In addition to the differences between fast and slow fibers just noted, other muscle proteins are also expressed in a fiber type-specific manner. Such proteins include SERCA, the three troponin subunits, tropomyosin, and C protein. The differential expression of SERCA isoforms (SERCA1 in fast-twitch muscle and SERCA2 in slow-twitch and cardiac muscle) contributes to the differences in the speed of relaxation between fast- and slow-twitch muscle. The activity of SERCA1 is greater than that of SERCA2. Therefore, Ca++ reuptake
into the SR occurs more quickly in fast muscles, and as a result, these fibers have a faster relaxation time. The differential expression of troponin and tropomyosin isoforms influences the dependency of contraction on Ca++. Slow fibers begin to develop tension at lower [Ca++] than fast fibers do. This differential sensitivity to Ca++ is related in part to the fact that the troponin C isoform in slow fibers has only a single low-affinity Ca++ binding site, whereas the troponin C of fast fibers has two low-affinity binding sites. Changes in the dependence of contraction on Ca++, however, are not restricted to differences in the troponin C isoforms. Differences in troponin T and tropomyosin isoforms are also found. Thus, regulation of the dependence of contraction on Ca++ is complex and involves contributions from multiple proteins on the thin filament.
|
| The activity pattern of a muscle is a major determinant of whether it adopts a fast-twitch or a slow-twitch phenotype. Thus, it is possible to convert a fast-twitch muscle to a slow-twitch muscle through cross-innervation or chronic electrical stimulation, as discussed later in this chapter. Ca++-dependent activation of the phosphatase calcineurin and the transcription factor "nuclear factor from activated T cells" (NFAT) have been implicated in this transition.
|
| MODULATION OF THE FORCE OF CONTRACTION
|
| page 244 |  | | page 245 |
| A simple means of increasing the force of contraction of a muscle is to recruit more muscle fibers. Because
all the muscle fibers within a motor unit are activated simultaneously, one recruits more muscle fibers by recruiting more motor units. As already noted, muscle fibers can be classified as fast twitch or slow twitch. The type of fiber is determined by its innervation. Because all fibers in a motor unit are innvervated by a single α motor neuron, all fibers within a motor unit are of the same type. Slow-twitch motor units tend to be small (100 to 500 muscle fibers) and are innervated by an α motor neuron that is easily excited. Fast-twitch motor units, by contrast, tend to be large (containing 1000 to 2000 muscle fibers) and are innervated by α motor neurons that are more difficult to excite. Thus, slow-twitch motor units tend to be recruited first. As more and more force is needed, fast-twitch motor units are recruited. The advantage of such a recruitment strategy is that the first muscle fibers recruited are those that have high resistance to fatigue. Moreover, the small size of slow-twitch motor units allows fine motor control at low levels of force. The process of increasing the force of contraction by recruiting additional motor units is termed spatial summation because one is "summing" forces from muscle fibers within a larger area of the muscle. This is in contrast to temporal summation, which is discussed later.
|
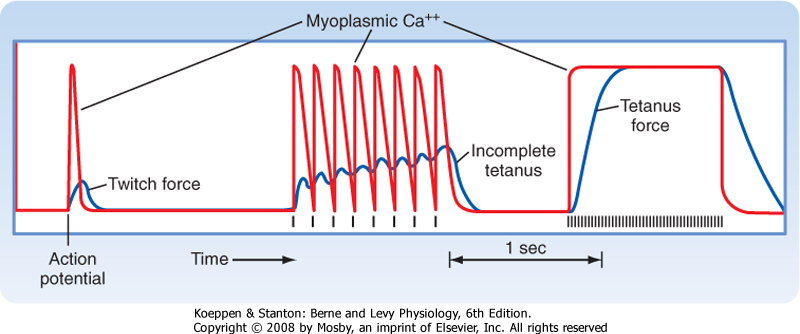
| Figure 12-16 Increasing the frequency of electrical stimulation of skeletal muscle results in an increase in the force of contraction. This is attributable to prolongation of the intracellular Ca++ transient and is termed tetany. Incomplete tetany results from initiation of another intracellular Ca++ transient before the muscle has completely relaxed. Thus, there is a summation of twitch forces. See text for details. |
| Action potentials in skeletal muscles are quite uniform and lead to the release of a reproducible pulse of Ca++ from the SR (Fig. 12-16). A single action potential releases sufficient Ca++ to cause a twitch contraction. However, the duration of this contraction is very short because Ca++ is very rapidly pumped back into the SR. If the muscle is stimulated a second time before the muscle is fully relaxed, the force of contraction increases (middle panel of Fig. 12-16). Thus, twitch forces are amplified as stimulus frequency increases. At a high level of stimulation, intracellular [Ca++] increases and is maintained throughout the period of stimulation (right panel of Fig. 12-16), and the amount of force developed greatly exceeds that seen during a
twitch. The response is termed tetany. At intermediate stimulus frequency, intracellular [Ca++] returns to baseline just before the next stimulus. However, there is gradual rise in force (middle panel of Fig. 12-16). This phenomenon is termed incomplete tetany. In both cases, the increased frequency of stimulation is said to produce a fusion of twitches.
|
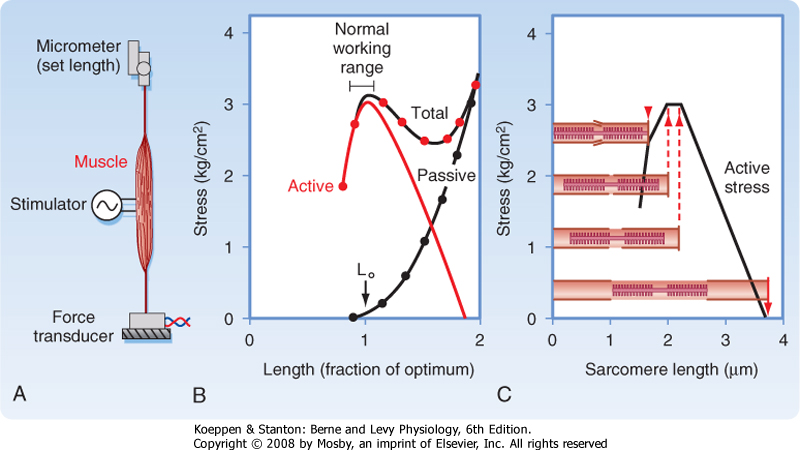
| It is hypothesized that the low force generation during a twitch, as compared with that seen during tetany, is due to the presence of a series elastic component in the muscle. Specifically, when the muscle is stretched a small amount shortly after initiation of the action potential, the muscle generates a twitch force that approximates the maximal tetanic force. This result, coupled with the observation that the size of the intracellular Ca++ transient during a twitch contraction is comparable to that seen during tetany, suggests that enough Ca++ is released into the myoplasm during a twitch to allow the actin-myosin interactions to produce maximal tension. However, the duration of the intracellular Ca++ transient during a twitch is sufficiently short that the contractile elements may not have enough time to fully stretch the series elastic components in the fiber and muscle. As a result, the measured tension is submaximal. Increasing the duration of the intracellular Ca++ transient, as occurs with tetany, provides the muscle with sufficient time to completely stretch the series elastic component and thereby results in expression of the full contractile force of the actin-myosin interactions (i.e., maximal tension). Partial stretching of the series elastic component (as might be expected during a single twitch), followed by restimulation of the muscle before complete relaxation, on the other hand, would be expected to yield an intermediate level of tension, similar to that seen with incomplete tetany. The location of the series elastic component in skeletal muscle is not known. One potential source is the myosin molecule itself. In addition, it is likely that there are other sources of the series elastic component, such as the connective tissue and titin.
|
| page 245 |  | | page 246 |
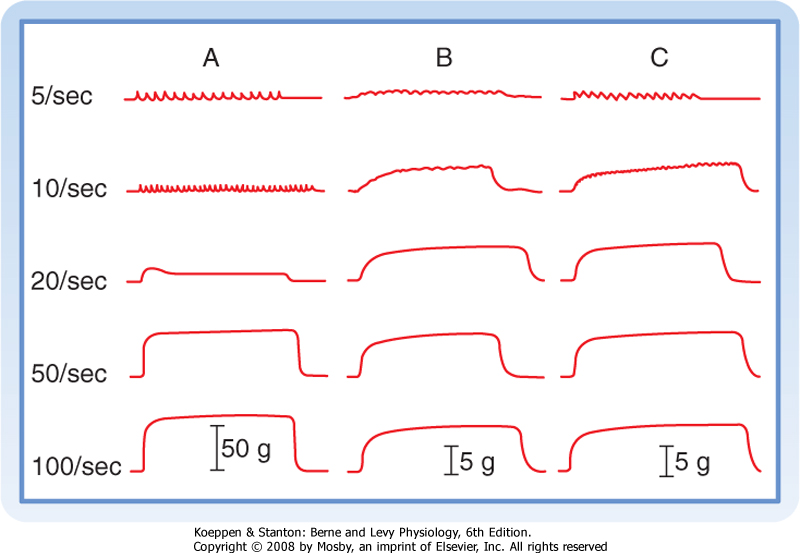
| Figure 12-17 Slow-twitch muscles tetanize at a lower stimulation frequency than that required for fast-twitch muscles. A, Fast-twitch motor unit in the gastrocnemius muscle. B, Slow-twitch motor unit in the gastrocnemius muscle. C, Slow-twitch muscle unit in the soleus muscle. The motor units were stimulated at the frequencies indicated on the left. The tension (in grams) generated during concentration is indicated by the vertical arrows. Note the large force generated by the fast-twitch motor unit (A). (From Montcastle V [ed]: Medical Physiology, 12th ed. St. Louis, Mosby, 1974.) |
| The stimulus frequency needed to produce tetany depends on whether the motor unit consists of slow or fast fibers (Fig. 12-17). Slow fibers can be tetanized at lower frequencies than is the case with fast fibers. The ability of slow-twitch muscle to tetanize at lower stimulation frequencies reflects, at least in part, the longer duration of contraction seen in slow fibers. As also illustrated in Figure 12-17, fast fibers develop a larger maximal force than slow fibers do because fast fibers are larger in diameter than slow fibers and there are more fibers in fast motor units than in slow motor units.
|
| MODULATION OF FORCE BY REFLEX ARCS
|
| Skeletal muscles contain sensory fibers (muscle spindles-also called intrafusal fibers) that run parallel to the skeletal muscle fibers. The muscle spindles assess the degree of stretch of the muscle, as well as the speed of contraction. In the stretch reflex, rapid stretching of the muscle (e.g., tapping the tendon) lengthens the spindles in the muscle and results in an increased frequency of action potentials in the afferent sensory neurons of the spindle. These afferent fibers in turn excite the α motor neurons in the spinal cord that innervate the stretched muscle. The result is that the reflex arc is a stretch-induced contraction of the muscle that does not require input from high centers in the brain. It should be noted that as the muscle shortens, efferent output to the spindle also occurs, thereby taking the slack out of the spindle and ensuring its ability to respond to stretch at all muscle lengths. By their action, muscle spindles provide feedback to the muscle in terms of its length and thus help maintain a joint at a given angle.
|
| Golgi tendon organs are located in the tendons of muscles and provide feedback regarding contraction of the muscle. The main component of the tendon organ is an elongated fascicle of collagen bundles that is in series with the muscle fibers and can respond to contractions of individual muscle fibers. A given tendon organ may attach to several fast-twitch or slow-twitch muscle fibers (or both) and sends impulses through Ib afferent nerve fibers in response to muscle contraction. The Ib afferent impulses enter the spinal cord, which can promote inhibition of α motor neurons to the contracting (and synergistic) muscles while promoting excitation of α motor neurons to antagonistic muscles. The inhibitory actions are mediated through interneurons in the cord that release an inhibitory transmitter to the α motor neuron and create an inhibitory postsynaptic potential (IPSP). The Ib afferent impulses are also sent to higher centers (including the motor cortex and cerebellum). It is hypothesized that feedback from the tendon organs in response to muscle contraction may smooth the progression of muscle contraction by limiting the recruitment of additional motor units. Interestingly, the response of the tendon organ is not linearly related to force but rather drops off at higher levels of force, which may facilitate the recruitment of motor units at higher levels of effort.
|
| The skeletal system supports the body in an erect posture with the expenditure of relatively little energy. Nonetheless, even at rest, muscles normally exhibit some level of contractile activity. Isolated (i.e., denervated) unstimulated muscles are in a relaxed state and are said to be flaccid. However, relaxed muscles in the body are comparatively firm. This firmness, or tone, is caused by low levels of contractile activity in some of the motor units and is driven by reflex arcs from the muscle spindles. Interruption of the reflex arc by sectioning the sensory afferent fibers will abolish this resting muscle tone. The tone in skeletal muscle is distinct from the "tone" in smooth muscle (see Chapter 14).
|
| ENERGY SOURCES DURING CONTRACTION
|
| Muscle cells convert chemical energy to mechanical energy. ATP is the energy source used for this conversion. The ATP pool in skeletal muscle is small and capable of supporting only a few contractions if not replenished. This pool, however, is continually replenished during contraction, as described later, such that even when the muscle fatigues, ATP stores are only modestly decreased.
|
| page 246 |  | | page 247 |
Muscle cells contain creatine phosphate, which is used to convert ADP to ATP and thus replenish the ATP store during muscle contraction. The creatine phosphate store represents the immediate high-energy source for replenishing the ATP supply in skeletal muscle, especially during intense exercise. The enzyme creatine phosphokinase (CPK) catalyzes the reaction

|
| Although much of the CPK is present in the myoplasm, a small amount is located in the thick filament (near the M line). The CPK in the thick filament may participate in the rapid resynthesis of ATP near the myosin heads during muscle contraction. The phosphate store created, however, is only about five times the size of the ATP store and thus cannot support prolonged periods of contraction (less than a minute of maximal muscle activity). Skeletal muscle fatigue during intense exercise is associated with depletion of the creatine phosphate store, although as described subsequently, this does not necessarily imply that the fatigue is caused by depletion of the creatine phosphate store. Because the CPK-catalyzed reaction shown above is reversible, the muscle cell replenishes the creatine phosphate pool during recovery from fatigue by using ATP synthesized through oxidative phosphorylation.
|
| Muscle cells contain glycogen, which can be metabolized during muscle contraction to provide glucose for oxidative phosphorylation and glycolysis, both of which will generate ATP to replenish the ATP store. Muscle cells can also take up glucose from blood, a process that is stimulated by insulin (see Chapter 38). The cytosolic enzyme phosphorylase releases glucose 1-phosphate residues from glycogen, which are then metabolized by a combination of glycolysis (in the cytosol) and oxidative phosphorylation (in the mitochondria) to yield the equivalent of 37 mol of ATP per mole of glucose 1-phosphate. Blood glucose yields 36 mol of ATP per mole of glucose because 1 ATP is used to phosphorylate glucose at the start of glycolysis. These ATP yields, however, are dependent on an adequate oxygen supply. Under anaerobic conditions, by contrast, metabolism of glycogen and glucose yields only 3 and 2 mol of ATP per mole of glucose 1-phosphate and glucose, respectively (along with 2 mol of lactate). As discussed later, muscle fatigue during prolonged exercise is associated with depletion of glycogen stores in the muscle.
|
| Fatty Acids and Triglycerides
|
| Fatty acids represent an important source of energy for muscle cells during prolonged exercise. Muscle cells contain fatty acids but can also take up fatty acids from blood. In addition, muscle cells can store triglycerides, which can be hydrolyzed when needed to produce fatty acids. The fatty acids are subjected to β oxidation within the mitochondria. For fatty acids to enter the mitochondria, however, they are converted to acyl-carnitine in the cytosol and then transported into the mitochondria, where they are converted to acyl-coenzyme A (CoA). Within the mitochondria, the acyl-CoA is subjected to β oxidation and yields acetyl-CoA, which then enters the citric acid cycle and ultimately produces ATP.
|
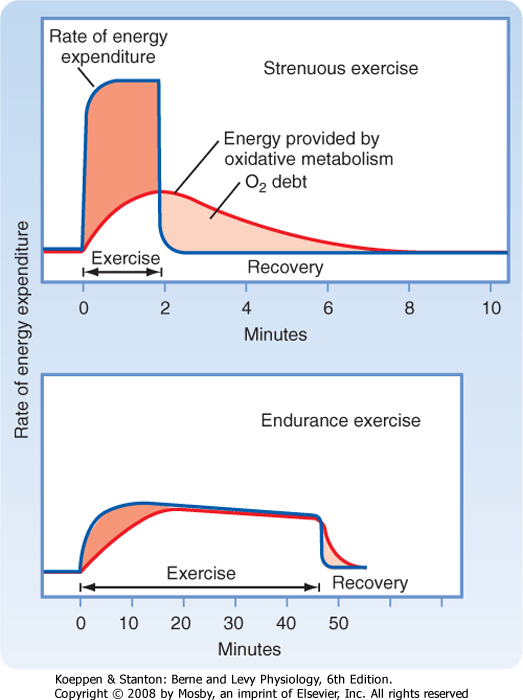
| Figure 12-18 An oxygen debt is incurred by exercising muscle when the rate of energy expenditure exceeds the rate of energy production by oxidative metabolism. Both strenuous (upper panel) and endurance exercise (lower panel) is shown. See text for details. |
| page 247 |  | | page 248 |
| If the energy demands of exercise cannot be met by oxidative phosphorylation, an oxygen debt is incurred. After completion of exercise, respiration remains above the resting level in order to "repay" this oxygen debt. The extra oxygen consumption during this recovery phase is used to restore metabolite levels (such as creatine phosphate and ATP) and to metabolize the lactate generated by glycolysis. The increased cardiac and respiratory work during recovery also contributes to the increased oxygen consumption seen at this time and explains why more O2 has to be "repaid" than was "borrowed." Some oxygen debt occurs even with low levels of exercise because slow oxidative motor units consume considerable ATP, derived from creatine phosphate or glycolysis, before oxidative metabolism can increase ATP production to meet steady-state requirements. The oxygen debt is much greater with strenuous exercise, when fast glycolytic motor units are used (Fig. 12-18). The oxygen debt is approximately
equal to the energy consumed during exercise minus that supplied by oxidative metabolism (i.e., the dark- and light-colored areas in Fig. 12-18 are roughly equal). As indicated earlier, the additional oxygen used during recovery from exercise represents the energy requirements for restoring normal cellular metabolite levels.
|
| The ability of muscle to meet energy needs is a major determinant of the duration of the exercise. However, fatigue is not the result of depletion of energy stores. Instead, metabolic byproducts seem to be important factors in the onset of fatigue. Fatigue may potentially occur at any of the points involved in muscle contraction, from the brain to the muscle cells, as well as in the cardiovascular and respiratory systems that maintain energy supplies (i.e., fatty acids and glucose) and O2 delivery to the exercising muscle.
|
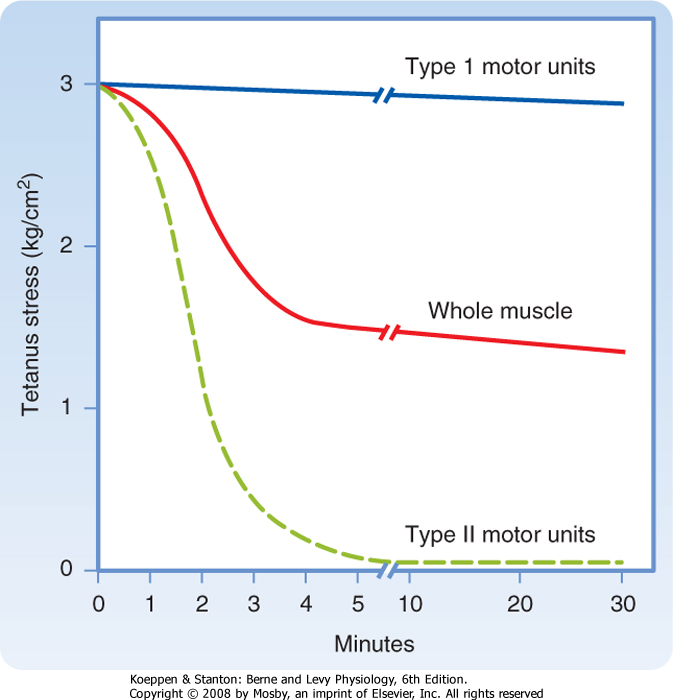
| Figure 12-19 A series of brief tetanic stimulations of skeletal muscle result in a rapid decrease in force (tetanic stress; "Whole muscle" in plot) that is attributable to fatigue of fast-twitch (type II) motor units in the muscle. Under these conditions, however, slow-twitch (type I) motor units are fatigue resistant. |
| Several factors have been implicated in muscle fatigue. During brief periods of tetany the oxygen supply to the muscle is adequate as long as the circulation is intact. However, the force/stress generated during these brief tetanic periods decays rapidly to a level that can be maintained for long periods (Fig. 12-19). This decay represents the rapid and almost total failure of the fast motor units. The decline in force/stress is paralleled by depletion of glycogen and creatine phosphate stores and accumulation of lactic acid. Importantly, the decline in force/stress occurs when the ATP pool is not greatly reduced, so the muscle fibers do not go into rigor. In contrast, the slow motor units are able to meet the energy demands of
fibers under this condition, and they do not exhibit significant fatigue, even after many hours. Evidently, some factor associated with energy metabolism can inhibit contraction (e.g., in the fast fibers), but this factor has not been clearly identified.
|
| During intense exercise, accumulation of Pi and lactic acid in the myoplasm accounts for muscle fatigue. The accumulation of lactic acid, to levels as high as 15 to 26 mM, decreases myoplasmic pH (from ≈7 to ≈6.2) and inhibits actin-myosin interactions. This decrease in pH reduces the sensitivity of the actin-myosin interaction to Ca++ by altering Ca++ binding to troponin C and by decreasing the maximum number of actin-myosin interactions. Fast-twitch fibers appear to be slightly more sensitive than slow-twitch muscle fibers to the effects of pH. Pi has also been implicated as an important factor in the development of fatigue during intense exercise inasmuch as phosphate concentrations can increase from around 2 mM at rest to nearly 40 mM in working muscle. Such an elevation in [Pi] can reduce tension by at least the following three different mechanisms: (1) inhibition of Ca++ release from the SR, (2) decrease in the sensitivity of contraction to Ca++, and (3) alteration in actin-myosin binding. A number of other factors, including glycogen depletion from a specialized compartment, a localized increase in [ADP], intracellular elevation of [K+], and generation of oxygen free radicals, have also been implicated in various forms of exercise-induced muscle fatigue. Finally, the central nervous system contributes to fatigue, especially the manner in which fatigue is perceived by the individual (see later).
|
| Regardless of whether the muscle is fatigued as a consequence of high-intensity exercise or prolonged exercise, the myoplasmic ATP level does not decrease substantially. Given the reliance of all cells on the availability of ATP to maintain viability, fatigue has been described as a protective mechanism to minimize the risk of muscle cell injury or death. Consequently, it is likely that skeletal muscle cells have developed redundant systems to ensure that ATP levels do not drop to dangerously low levels and hence risk the viability of the cell.
|
| Most persons tire and cease exercise long before the motor unit fatigues. General physical fatigue may be defined as a homeostatic disturbance produced by work. The basis for the perceived discomfort (or even pain) probably involves many factors. These factors may include a decrease in plasma glucose levels and accumulation of metabolites. Motor system function in the central nervous system is not impaired. Highly motivated and trained athletes can withstand the discomfort of fatigue and will exercise to the point at which some motor unit fatigue occurs. Part of the enhanced performance observed after training involves motivational factors.
|
| page 248 |  | | page 249 |
| Skeletal muscle fibers differentiate before they are innervated, and some neuromuscular junctions are
formed well after birth. Before innervation, the muscle fibers physiologically resemble slow (type I) cells. Acetylcholine receptors are distributed throughout the sarcolemma of these uninnervated cells and are supersensitive to that neurotransmitter. An end plate is formed when the first growing nerve terminal establishes contact with a muscle cell. The cell forms no further association with nerves, and receptors to acetylcholine become concentrated in the end plate membranes. Cells innervated by a small motor neuron form slow (type I) oxidative motor units. Fibers innervated by large motor nerves develop all the characteristics of fast (type II) motor units. Innervation produces major cellular changes, including synthesis of the fast and slow myosin isoforms, which replace embryonic or neonatal variants. Thus, muscle fiber type is determined by the nerves that innervate the fiber.
|
|
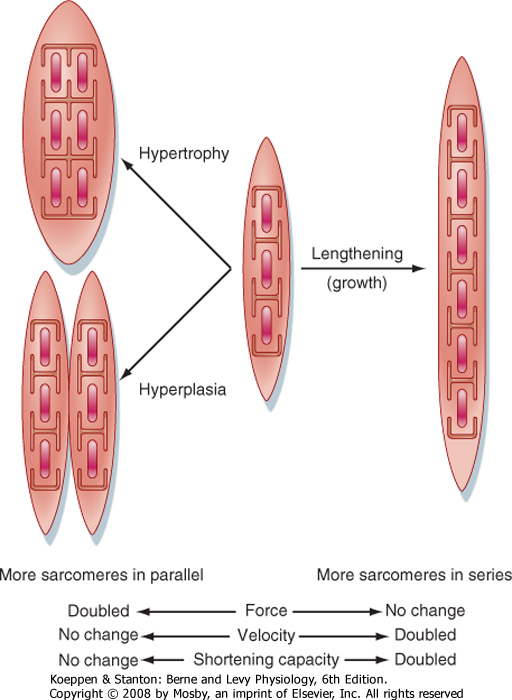
| Figure 12-20 Effects of growth on the mechanical output of a muscle cell. Typically, skeletal muscle cell growth involves either lengthening (adding more sarcomeres to the ends of the muscle fibers) or increasing muscle fiber diameter (hypertrophy as a result of the addition of more myofilaments/myofibrils in parallel within the muscle fiber). The formation of new muscle fibers is called hyperplasia, and it is infrequent in skeletal muscle. |
| An increase in muscle strength and size occurs during maturation. As the skeleton grows, the muscle cells lengthen. Lengthening is accomplished by the formation of additional sarcomeres at the ends of the
muscle cells (Fig. 12-20), a process that is reversible. For example, the length of a cell decreases when terminal sarcomeres are eliminated, which can occur when a limb is immobilized with the muscle in a shortened position or when an improperly set fracture leads to a shortened limb segment. Changes in muscle length affect the velocity and extent of shortening but do not influence the amount of force that can be generated by the muscle. The gradual increase in strength and diameter of a muscle during growth is achieved mainly by hypertrophy. Doubling the myofibrillar diameter by adding more sarcomeres in parallel (hypertrophy, for example) may double the amount of force generated but has no effect on the maximal velocity of shortening.
|
| Skeletal muscles have a limited ability to form new fibers (hyperplasia). These new fibers result from differentiation of satellite cells that are present in the tissues. However, major cellular destruction leads to replacement by scar tissue.
|
| Muscles must not only be used to maintain normal growth and development but must also experience a load. Muscles immobilized in a cast lose mass. In addition, space flight exposes astronauts to a microgravity environment that mechanically unloads their muscles. Such unloading leads to rapid loss of muscle mass (i.e., atrophy) and weakness. Atrophy appears to involve both inhibition of protein synthesis and stimulation of protein degradation.
|
| Muscles that frequently contract to support the body typically have a high number of slow (type I) oxidative motor units. These slow motor units atrophy more rapidly than the fast (type II) motor units during prolonged periods of unloading. This atrophy of slow motor units is associated with a decrease in maximal tetanic force, but an increase in maximal shortening velocity. The increase in velocity is correlated with expression of the fast myosin isoform in these fibers. An important aspect of space medicine is the design of exercise programs that minimize such phenotypic changes during prolonged space flight.
|
| page 249 |  | | page 250 |
| Figure 12-21 Molecular signaling pathways contributing to atrophy of skeletal muscle. A decrease in activity of the PI3K/Akt pathway has been implicated in a variety of muscular atrophies and results in stimulation of proteolysis (through activation of the protease caspase 3 and expression of atrophy genes such as the ubiquitin ligase atrogin), decreased protein synthesis (through activation of an inhibitor of translation, 4E-BP1), and limited nuclear death (apoptosis). Decreased contractile activity also results in release of the ubiquitin ligase MuRF2 from titin and activation of the transcription factor NF-κB, both of which contribute to gene regulation of atrophy. (From Kandarian SC, Jackman RW: Muscle Nerve 33:155-165, 2006.) |
| One factor thought to contribute to the decreased protein synthesis and increased protein degradation during periods of mechanical inactivity is the release of a ubiquitin ligase (MuRF2) from titin (Fig. 12-21). Specifically, MuRF2 inhibits transcription by exporting a transcription factor (serum response factor [SRF]) from the nucleus into the myoplasm. MuRF2 also promotes protein degradation through ubiquitination (see Chapter 1). In addition to the actions of MuRF2, atrophy is also thought to involve inhibition of a phosphatidylinositol-3-kinase (PI3K) signaling cascade. Inhibition of PI3K and the serine/threonine kinase Akt appears to contribute to the decrease in protein synthesis by inhibiting eukaryotic translation initiation factor 4E. Decreased activity of PI3K can also stimulate proteolysis through activation of caspase 3 or through ubiquitination (or both). The increased ubiquitination is thought to be result from increased expression of a ubiquitin ligase (atrogin) and would complement the increased ubiquitination resulting from release of the ubiquitin ligase MuRF2 from titin, as described earlier. |
 |
| Testosterone is a major factor responsible for the greater muscle mass in males because it has myotrophic action as well as androgenic (masculinization) effects (see Chapter 43). A variety of synthetic molecules, called anabolic steroids, have been designed to enhance muscle growth while minimizing their androgenic action. These drugs are widely used by bodybuilders and athletes in sports in which strength is important. The doses are typically 10- to 50-fold greater than might be prescribed therapeutically for individuals with impaired hormone production. Unfortunately, none of these compounds lack androgenic effects. Hence, at the doses used, they induce serious hormone disturbances, including depressed testosterone production. A major issue is whether these drugs do in fact increase muscle and athletic performance in individuals with normal circulating levels of testosterone. After some 4 decades of use, the scientific facts remain uncertain, and most experimental studies in animals have not documented any significant effects on muscle development. Reports in humans remain
controversial. Proponents claim increases in strength that provide the edge in world-class performance. Critics argue that these increases are largely placebo effects associated with expectations and motivational factors. The public debate on abuse of anabolic steroids has led to their designation as controlled substances, along with opiates, amphetamines, and barbiturates.
|
| DENERVATION, REINNERVATION, AND CROSS-INNERVATION
|
| As already noted, innervation is critical to the skeletal muscle phenotype. If the motor nerve is cut, muscle fasciculation occurs. Fasciculation is characterized by small, irregular contractions caused by release of acetylcholine from the terminals of the degenerating distal portion of the axon. Several days after denervation, muscle fibrillation begins. Fibrillation is characterized by spontaneous, repetitive contractions. At this time, the cholinergic receptors have spread out over the entire cell membrane, in effect reverting to their preinnervation embryonic arrangement. The muscle fibrillations reflect supersensitivity to acetylcholine. Muscles also atrophy, with a decrease in the size of the muscle and its cells. Atrophy is progressive in humans, with degeneration of some cells 3 or 4 months after denervation. Most of the muscle fibers are replaced by fat and connective tissue after 1 to 2 years. These changes can be reversed if reinnervation occurs within a few months. Reinnervation is normally achieved by growth of the peripheral stump of motor nerve axons along the old nerve sheath.
|
| page 250 |  | | page 251 |
| Reinnervation of formerly fast (type II) fibers by a small motor axon causes that cell to redifferentiate into a slow (type I) fiber, and vice versa. This suggests that large and small motor nerves differ qualitatively and that the nerves have specific "trophic" effects on the muscle fibers. This "trophic" effect reflects the rate of fiber stimulation. For example, stimulation via electrodes implanted in the muscle can lessen denervation atrophy. More strikingly, chronic low-frequency stimulation of fast motor units causes these units to be converted to slow units. Some conversion toward a typical fast-fiber phenotype can occur when the frequency of contraction in slow units is greatly decreased by reducing the excitatory input. Excitatory input can be reduced by sectioning the appropriate spinal or dorsal root or by severing the tendon, which functionally inactivates peripheral mechanoreceptors.
|
| The frequency of contraction determines fiber development and phenotype through changes in gene expression and protein synthesis. Fibers that undergo frequent contractile activity form many mitochondria and synthesize the slow isoform of myosin. Fibers innervated by large, less excitable axons contract infrequently. Such relatively inactive fibers typically form few mitochondria and have large concentrations of glycolytic enzymes. The fast isoform of myosin is synthesized in such cells.
|
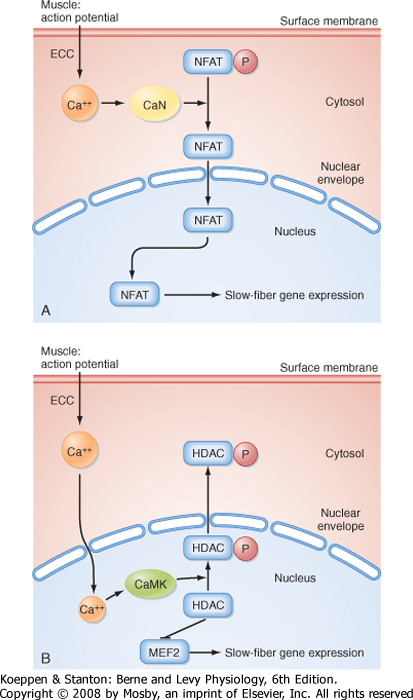
| The transcription factor nuclear factor from activated T cells (NFAT) has been implicated in this transition from fast-twitch to slow-twitch muscle (Fig. 12-22, A). Specifically, it appears that stimulation of adult fast-twitch muscle cells at a frequency consistent with slow-twitch muscle cells can activate the Ca++-dependent phosphatase calcineurin, which in turn can dephosphorylate NFAT and result in translocation of NFAT from the myoplasm to the nucleus, followed by the transcription of slow-twitch muscle genes (and inhibition of fast-twitch muscle genes). Consistent with this mechanism, expression of constitutively active NFAT in fast-twitch muscle promotes the expression of slow-twitch myosin while inhibiting the expression of fast-twitch myosin. The transcription factor myocyte enhancing factor 2 (MEF2) has also been implicated in this transition from fast-twitch to slow-twitch muscle (Fig. 12-22, B). Activation of MEF2 is thought to result from Ca++-calmodulin-dependent phosphorylation of an inhibitor of MEF2 (viz., histone deacetylase [HDAC]). |
 |
|
| Figure 12-22 Molecular signaling pathways contributing to the transition from fast-twitch muscle to slow-twitch muscle. Chronic electrical stimulation of a fast-twitch muscle in a pattern consistent with a slow-twitch muscle results in development of the slow-twitch muscle phenotype because of dephosphorylation of the transcription factor NFAT by the Ca++-calmodulin-dependent protein phosphatase calcineurin (CaN); this in turn results in nuclear translocation of NFAT and expression of slow-twitch muscle fiber genes (A). Activation of the transcription factor MEF2 also appears to contribute to this fiber type transition (B), with activation of MEF2 involving Ca++-calmodulin-dependent phosphorylation of an inhibitor (histone deacetylase [HDAC]). (From Liu Y et al: J Muscle Res Cell Motil 26:13-21, 2005.) |
| page 251 |  | | page 252 |
|
Table 12-3.
Effects of Exercise |
| Type of Training | Example | Major Adaptive Response |
| Learning/coordination skills | Typing | Increased rate and accuracy of motor units (central nervous system) |
| Endurance (submaximal, sustained efforts) | Marathon running | Increased oxidative capacity in all involved motor units with limited cellular hypertrophy |
| Strength (brief, maximal efforts) | Weightlifting | Hypertrophy and enhanced glycolytic capacity of the motor units used |
| Intracellular [Ca++] appears to play an important role in expression of the slow myosin isoform. Slow-twitch muscle fibers have a higher resting level of intracellular Ca++ than fast-twitch muscle does. In addition, chronic electrical stimulation of fast-twitch muscle is accompanied by a 2.5-fold increase in resting myoplasmic [Ca++] that precedes the increased expression of slow-twitch myosin and decreased expression of fast-twitch myosin. Similarly, chronic elevation of
intracellular Ca++ (approximately fivefold) in muscle cells expressing fast-twitch myosin induces a change in gene expression from the fast muscle myosin isoform to the slow myosin isoform within 8 days. An increase in citrate synthetase activity (an indicator of oxidative
capacity) and a decrease in lactate dehydrogenase activity (an indicator of glycolytic capacity) accompany this Ca++-dependent transition from fast-twitch to slow-twitch myosin. These Ca++-dependent changes are reversible by lowering intracellular [Ca++].
|
| Exercise physiologists identify three categories of training regimens and responses: learning, endurance, and strength training (Table 12-3). Typically, most athletic endeavors involve elements of all three. The learning aspect of training involves motivational factors, as well as neuromuscular coordination. This aspect of training does not involve adaptive changes in the muscle fibers per se. However, motor skills can persist for years without regular training, unlike the responses of muscle cells to exercise.
|
| All healthy persons can maintain some level of continuous muscular activity that is supported by oxidative metabolism. This level can be greatly increased by a regular exercise regimen that is sufficient to induce adaptive responses. The adaptive response of skeletal muscle fibers to endurance exercise is mainly the result of an increase in the oxidative metabolic capacity of the motor units involved. This demand places an increased load on the cardiovascular and respiratory systems and increases the capacity of the heart and respiratory muscles. The latter effects are responsible for the principal health benefits associated with endurance exercise.
|
| Muscle strength can be increased by regular massive efforts that involve most motor units. Such efforts recruit fast glycolytic motor units, as well as slow oxidative motor units. During these efforts, blood supply to the working muscles may be interrupted as tissue pressures rise above intravascular pressure. The reduced blood flow limits the duration of the contraction. Regular maximal-strength exercise, such as weightlifting, induces the synthesis of more myofibrils and hence hypertrophy of the active muscle cells. The increased stress also induces the growth of tendons and bones.
|
| Endurance exercise does not cause fast motor units to become slow, nor does maximal muscular effort produce a shift from slow to fast motor units. Thus, any practical exercise regimen, when superimposed on normal daily activities, probably does not alter muscle fiber phenotype.
|
| DELAYED-ONSET MUSCLE SORENESS
|
| Activities such as hiking or, in particular, downhill running, in which contracting muscles are stretched and lengthened too vigorously, are followed by more pain and stiffness than after comparable exercise that does not involve vigorous muscle stretching and lengthening (e.g., cycling). The resultant dull, aching pain develops slowly and reaches its peak within 24 to 48 hours. The pain is associated with reduced range of motion, stiffness, and weakness of the affected muscles. The prime factors that cause the pain are swelling and inflammation from injury to muscle cells, most commonly near the myotendinous junction. Fast type II motor units are affected more than type I motor units because the maximal force is highest in large cells, where the loads imposed are some 60% greater than the maximal force that the cells can develop. Recovery is slow and depends on regeneration of the injured sarcomeres.
|
| BIOPHYSICAL PROPERTIES OF SKELETAL MUSCLE
|
| The molecular mechanisms of muscle contraction described earlier underlie and are responsible for the biophysical properties of muscle. Historically, these biophysical properties were well described before elucidation of the molecular mechanisms of contraction. They remain important ways of describing muscle function.
|
| Length-Tension Relationship
|
| When muscles contract, they generate force (often measured as tension or stress) and decrease in length. When studying the biophysical properties of muscle, one of these parameters is usually held constant while the other is measured after an experimental maneuver. Accordingly, an isometric contraction is one in which muscle length is held constant, and the force generated during the contraction is then measured. An isotonic contraction is one in which the force (or tone) is held constant, and the change in length of the muscle is then measured.
|
| page 252 |  | | page 253 |
| Figure 12-23 Length-tension relationship in skeletal muscle. A, Experimental setup in which maximal isometric tetanic tension is measured at various muscle lengths. B, How active tension was calculated at various muscle lengths (i.e., by subtracting passive tension from total tension at each muscle length). C, Plot of active tension as a function of muscle length, with the predicted overlap of thick and thin filaments at selected points. |
| Figure 12-24 Force-velocity relationship of skeletal muscle. The experimental setup is shown on the right. The initial muscle length was kept constant, but the amount of weight that the muscle had to lift during tetanic stimulation varied. Muscle-shortening velocity while lifting these various amounts of weight was measured. See text for details. |
| When a muscle at rest is stretched, it resists stretch by a force that increases slowly at first and then more rapidly as the extent of stretch increases (Fig. 12-23). This purely passive property is due to the elastic tissue in the muscle. If the muscle is stimulated to contract at these various lengths, a different relationship is obtained. Specifically, contractile force increases as muscle length is increased up to a point (designated L0 to indicate optimal length). As the muscle is stretched beyond L0, contractile force decreases. This length-tension curve is consistent with the sliding filament theory. At a very long sarcomere length (3.7 μm), actin filaments no longer overlap with myosin filaments,
so there is no contraction. As muscle length is decreased toward L0, the amount of overlap increases, and contractile force progressively increases. As sarcomere length decreases below 2 μm, the thin filaments collide in the middle of the sarcomere, and the actin-myosin interaction is disturbed and hence contractile force decreases. Note that for construction of the length-tension curves, muscles were maintained at a given length, and then contractile force was measured (i.e., isometric contraction). Thus, the length-tension relationship supports the sliding filament theory of muscle contraction described previously.
|
| Force-Velocity Relationship
|
| page 253 |  | | page 254 |
| The velocity at which a muscle shortens is strongly dependent on the amount of force that the muscle must develop (Fig. 12-24). In the absence of any load, the shortening velocity of the muscle is maximal
(denoted as V0). V0 corresponds to the maximal cycling rate of the cross-bridges (i.e., it is proportional to the maximal rate of energy turnover [ATPase activity] by myosin). Thus, V0 for fast-twitch muscle is higher than that for slow-twitch muscle. Increasing the load decreases the velocity of muscle shortening until, at maximal load, the muscle cannot lift the load and hence cannot shorten (zero velocity). Further increases in load result in stretching the muscle (negative velocity). The maximal isometric tension (i.e., force at which shortening velocity is zero) is proportional to the number of active cross-bridges between actin and myosin, and it is usually greater for fast-twitch motor units (given the larger diameter of fast-twitch muscle fibers and greater number of muscle fibers in a typical fast-twitch motor unit). The curve labeled "power-stress curve" reflects the rate of work done at each load and shows that the maximal rate of work was done at a submaximal load (viz., when the force of contraction was approximately 30% of the maximal tetanic tension). The latter curve was calculated simply by multiplying the x and y coordinates and then plotting the product as a function of the x coordinate.
|
| page 254 |  | | page 255 |
- Skeletal muscle is composed of numerous muscle cells (muscle fibers) that are typically 10 to 80 μm in diameter and up to 25 cm in length. Striations are apparent in skeletal muscle and are due to the highly organized arrangement of thick and thin filaments in the myofibrils of skeletal muscle fibers. The sarcomere is a contractile unit in skeletal muscle. Each sarcomere is approximately 2 μm in length at rest and is bounded by two Z lines. Sarcomeres are arranged in series along the length of the myofibril. Thin filaments, containing actin, extend from the Z line toward the center of the sarcomere. Thick filaments, containing myosin, are positioned in the center of the sarcomere and overlap the actin thin filaments. Muscle contraction results from the Ca++-dependent interaction of myosin and actin, with myosin pulling the thin filaments toward the center of the sarcomere.
- Contraction of skeletal muscle is under control of the central nervous system (i.e., voluntary). Motor centers in the brain control the activity of α motor neurons in the ventral horns of the spinal cord. These α motor neurons, in turn, synapse on skeletal muscle fibers. Although each skeletal muscle fiber is innervated by only one motor neuron, a motor neuron innervates several muscle fibers within the muscle. A motor unit refers to all the muscle fibers innervated by a single motor neuron.
- The motor neuron initiates contraction of skeletal muscle by producing an action potential in the muscle fiber. As the action potential passes down the T tubules of the muscle fiber, dihydropyridine receptors (DHPRs) in the T tubules undergo conformational changes that result in the opening of neighboring SR Ca++ channels called ryanodine receptors (RYRs), which then release Ca++ to the myoplasm from the SR. The increase in myoplasmic Ca++ promotes muscle contraction by exposing myosin binding sites on the actin thin filaments (a process that involves binding of Ca++ to troponin C, followed by movement of tropomyosin toward the groove in the thin filament). Myosin cross-bridges then appear to undergo a ratchet action, with the thin filaments pulled toward the center of the sarcomere and contracting the skeletal muscle fiber. Relaxation of the muscle follows as myoplasmic Ca++ is resequestered by Ca++-ATPase (SERCA) in the SR.
- The force of contraction can be increased by activating more motor neurons (i.e., recruiting more muscle fibers) or by increasing the frequency of action potentials in the muscle fiber, which produces tetany. The increased force during tetanic contractions is due to prolonged elevation of intracellular [Ca++].
- The two basic types of skeletal muscle fibers are distinguished on the basis of their speed of contraction (i.e., fast twitch versus slow twitch). The difference in speed of contraction is attributed to the expression of different myosin isoforms that differ in myosin ATPase activity. In addition to the difference in myosin ATPase activity, fast- and slow-twitch muscles also differ in metabolic activity, fiber diameter, motor unit size, sensitivity to tetany, and recruitment pattern.
- Typically, slow-twitch muscles are recruited before fast-twitch muscle fibers because of the greater excitability of motor neurons innervating slow-twitch muscles. The high oxidative capacity of slow-twitch muscle fiber supports sustained contractile activity. Fast-twitch muscle fibers, in contrast, tend to be large and typically have low oxidative capacity and high glycolytic capacity. The fast-twitch motor units are thus best suited for short periods of activity when high levels of force are required.
- Fast-twitch muscle fibers can be converted to slow-twitch muscle fibers (and vice versa), depending on the stimulation pattern. Chronic electrical stimulation of a fast-twitch muscle results in the expression of slow-twitch myosin and decreased expression of fast-twitch myosin, along with an increase in oxidative capacity. The mechanism or mechanisms underlying this change in gene expression are unknown but appear to be secondary to an elevation in resting intracellular [Ca++]. The Ca++-dependent phosphatase calcineurin and the transcription factor NFAT have been implicated in this transition from the fast-twitch to the slow-twitch phenotype. Ca++-calmodulin-dependent kinase and the transcription factor MEF2 may also participate in the phenotype transition.
- Skeletal muscle fibers atrophy after denervation. Muscle fibers depend on the activity of their motor nerves for maintenance of the differentiated phenotype. Reinnervation by axon growth along the original nerve sheath can reverse these changes. Skeletal muscle has a limited capacity to replace cells lost as a result of trauma or disease. Inhibition of the PI3K/Akt signaling pathways appears to contribute to the decreased rate of protein synthesis and increased rate of protein degradation observed during atrophy. The increased protein degradation during atrophy is attributed to increases in both protease activity (e.g., activation of caspase 3) and ubiquitination (through elevated levels of ubiquitin ligases). During disuse-induced atrophy, release of the ubiquitin ligase MuRF2 appears to contribute to decreased transcription and increased protein degradation.
- Skeletal muscle exhibits considerable phenotypic plasticity. Normal growth is associated with cellular hypertrophy caused by the addition of more myofibrils and more sarcomeres at the ends of the cell to match skeletal growth. Strength training induces cellular hypertrophy, whereas endurance training increases the oxidative capacity of all involved motor units. Training regimens are not able to alter fiber type or the expression of myosin isoforms.
- Muscle fatigue during exercise is not due to depletion of ATP. The mechanism or mechanisms underlying exercise-induced fatigue are not known, although the accumulation of various metabolic products (lactate, Pi, ADP) has been implicated. Given the importance of preventing depletion of myoplasmic ATP, which would affect the viability of the cell, it is likely that multiple mechanisms may have been developed to induce fatigue and hence lower the rate of ATP hydrolysis before risking injury/death of the skeletal muscle cell.
- When the energy demands of an exercising muscle cannot be met by oxidative metabolism, an oxygen debt is incurred. Increased breathing during the recovery period after exercise reflects this O2 debt. The greater the reliance on anaerobic metabolism to meet the energy requirements of muscle contraction, the greater the O2 debt.
|
 |
|