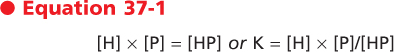| SECTION EIGHT THE ENDOCRINE REPRODUCTIVE SYSTEMS
|
| page 651 |  | | page 652 |
| page 652 |  | | page 653 |
| 37 Introduction to the Endocrine System
|
| The ability of cells to communicate with each other represents an underpinning of human biology. As discussed in Chapter 3, cell-to-cell communication exists at various levels of complexity and distance. Endocrine signaling involves (1) the regulated secretion of an extracellular signaling molecule, called a hormone, into the extracellular fluid; (2) diffusion of the hormone into the vasculature and its circulation throughout the body; and (3) diffusion of the hormone out of the vascular compartment into the extracellular space and binding to a specific receptor within cells of a target organ. Because of the spread of hormones throughout the body, one hormone often regulates the activity of several target organs. Conversely, cells frequently express receptors for multiple hormones.
|
The endocrine system is a collection of glands whose function is to regulate multiple organs within the body to (1) meet the growth and reproductive needs of the organism and (2) respond to fluctuations within the internal environment, including various types of stress. The endocrine system comprises the following major glands (Fig. 37-1):
- Endocrine pancreas
- Parathyroid glands
- Pituitary gland (in association with hypothalamic nuclei)
- Thyroid gland
- Adrenal glands
- Gonads (testes or ovaries)
|
| These endocrine glands synthesize and secrete bioactive hormones and, with the exception of gonads, which perform both endocrine and gametogenic functions, are dedicated to hormone production (Table 37-1). A transitory organ, the placenta, also performs a major endocrine function.
|
| In addition to dedicated endocrine glands, there are endocrine cells within organs whose primary function is not endocrine (Table 37-1). These include cells within the heart that produce atrial natriuretic peptide, liver cells that produce insulin-like growth factor type I (IGF-I), cells within the kidney that produce erythropoietin, and numerous cell types within the gastrointestinal tract that produce gastrointestinal hormones. There also exist collections of cell bodies (called nuclei) within the hypothalamus that secrete peptides, called neurohormones, into capillaries associated with the pituitary gland.
|
| A third arm of the endocrine system is represented by numerous cell types that express intracellular enzymes, ectoenzymes, or secreted enzymes that modify inactive precursors or less active hormones into highly active hormones (Table 37-1). An example is the generation of angiotensin II from the inactive polypeptide angiotensinogen by two subsequent proteolytic cleavages (see Chapter 42). Another example is activation of vitamin D by two subsequent hydroxylation reactions in the liver and kidney to produce the highly bioactive hormone 1,25-dihydroxyvitamin D (vitamin D).
|
| CONFIGURATION OF FEEDBACK LOOPS WITHIN THE ENDOCRINE SYSTEM
|
| The predominant mode of a closed feedback loop among endocrine glands is negative feedback. In a negative-feedback loop, "hormone A" acts on one or more target organs to induce a change (either a decrease or increase) in circulating levels of "component B," and the change in component B then inhibits secretion of hormone A. Negative-feedback loops confer stability by keeping a physiological parameter (e.g., blood glucose) within a normal range. There are also a few examples of positive feedback in endocrine regulation. A positive closed feedback loop, in which hormone X increases levels of component Y and component Y stimulates secretion of hormone X, confers instability. Under the control of positive-feedback loops, something has "got to give." For example, positive-feedback loops control processes that lead to rupture of a follicle through the ovarian wall or expulsion of a fetus from the uterus.
|
| page 653 |  | | page 654 |
| Figure 37-1 Glands of the endocrine system. |
| Figure 37-2 Physiological response-driven and endocrine axis-driven negative-feedback loops. |
| There are two basic configurations of negative-feedback loops within the endocrine system: a physiological response-driven feedback loop (referred to simply as "response-driven feedback") and an endocrine axis-driven feedback loop (Fig. 37-2). The response-driven feedback loop is observed in endocrine glands that control blood glucose levels (pancreatic islets), blood Ca++ and Pi levels (parathyroid glands, kidney), blood osmolarity and volume (hypothalamus/posterior pituitary), and blood Na+, K+, and H+ (zona glomerulosa of the adrenal cortex and atrial cells). In the response-driven configuration, secretion of a hormone is stimulated or inhibited by a change in the level of a specific extracellular parameter (e.g., an increase in blood glucose stimulates insulin secretion). Altered
hormone levels lead to changes in the physiology of target organs (e.g., decreased hepatic gluconeogenesis, increased uptake of glucose by muscle) that directly regulate the parameter (i.e., blood glucose) in question. The change in the parameter (i.e., decreased blood glucose) then inhibits further secretion of the hormone (i.e., insulin secretion drops as blood glucose falls).
|
| page 654 |  | | page 655 |
|
Table 37-1.
Hormones and Their Sites of Production in Nonpregnant Adults |
| Gland | Hormone |
| Hormones Synthesized and Secreted by Dedicated Endocrine Glands |
| Pituitary gland | Growth hormone (GH)
Prolactin
Adrenocorticotropic hormone (ACTH)
Thyroid-stimulating hormone (TSH)
Follicle-stimulating hormone (FSH)
Luteinizing hormone (LH) |
| Thyroid gland | Tetraiodothyronine (T4; thyroxine)
Triiodothyronine (T3)
Calcitonin |
| Parathyroid glands | Parathyroid hormone (PTH) |
| Islets of Langerhans (endocrine pancreas) | Insulin
Glucagon
Somatostatin |
| Adrenal gland | Epinephrine
Norepinephrine
Cortisol
Aldosterone
Dehydroepiandrosterone sulfate (DHEAS) |
| Ovaries | Estradiol-17β
Progesterone
Inhibin |
| Testes | Testosterone
Antimüllerian hormone (AMH)
Inhibin |
| Hormones Synthesized in Organs with a Primary Function Other Than Endocrine |
| Brain (hypothalamus) | Antidiuretic hormone (ADH; vasopressin)
Oxytocin
Corticotropin-releasing hormone (CRH)
Thyrotropin-releasing hormone (TRH)
Gonadotropin-releasing hormone (GnRH)
Growth hormone-releasing hormone (GHRH)
Somatostatin
Dopamine |
| Brain (pineal gland) | Melatonin |
| Heart | Atrial natriuretic peptide (ANP) |
| Kidney | Erythropoietin |
| Adipose tissue | Leptin
Adiponectin |
| Stomach | Gastrin
Somatostatin
Ghrelin |
| Intestines | Secretin
Cholecystokinin
Glucagon-like peptide-1 (GLP-1)
Glucagon-like peptide-2 (GLP-2)
Glucose-dependent insulinotropic peptide (GIP; gastrin inhibitory peptide)
Motilin |
| Liver | Insulin-like growth factor type I (IGF-I) |
| Hormones Produced to a Significant Degree by Peripheral Conversion |
| Lungs | Angiotensin II |
| Kidney | 1,25-Dihydroxyvitamin D (vitamin D) |
| Adipose, mammary glands, other organs | Estradiol-17β |
| Liver, sebaceous gland, other organs | Testosterone |
| Genital skin, prostate, other organs | 5-Dihydrotestosterone (DHT) |
| Many organs | T3 |
| Much of the endocrine system is organized into endocrine axes, with each axis consisting of the hypothalamus and the pituitary and peripheral endocrine glands (Fig. 37-2). Thus, the endocrine axis-driven feedback loop involves a three-tiered configuration. The first tier is represented by hypothalamic neuro-endocrine neurons that secrete releasing hormones. Releasing hormones stimulate (or, in a few cases, inhibit) the production and secretion of tropic hormones from the pituitary gland (second tier). Tropic hormones stimulate the production and secretion of hormones from peripheral endocrine glands (third tier). The peripherally produced hormones, namely, thyroid hormone, cortisol, sex steroids, and IGF-I, typically have pleiotropic actions (e.g., multiple phenotypic effects) on numerous cell types. However, in endocrine axis-driven feedback, the primary feedback loop involves feedback inhibition of pituitary tropic
hormones and hypothalamic releasing hormones by the peripherally produced hormone. In contrast to response-driven feedback, the physiological responses to the peripherally produced hormone play only a minor role in regulation of feedback within endocrine axis-driven feedback loops.
|
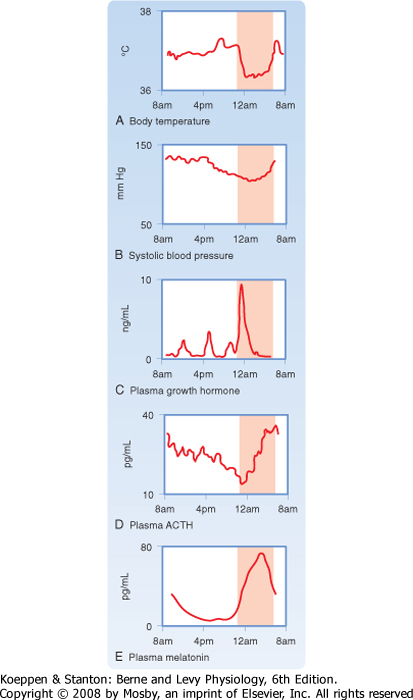
| An important aspect of the endocrine axes is the ability of descending and ascending neuronal signals to modulate release of the hypothalamic releasing hormones and thereby control the activity of the axis. A major neuronal input to releasing hormone-secreting neurons comes from another region of the hypothalamus called the suprachiasmatic nucleus (SCN). SCN neurons impose a daily rhythm, called a circadian rhythm, on the secretion of hypothalamic releasing hormones and the endocrine axes that they control (Fig. 37-3). SCN neurons represent an intrinsic circadian clock, as evidenced by the fact that they demonstrate a spontaneous peak of electrical activity at the same time every 24 to 25 hours. The 24- to 25-hour cycle can be "entrained" by the normal environmental light-dark cycle created by the earth's rotation such that the periodicity of the clock appears to be environmentally controlled (Fig. 37-4). Neural input is generated from specialized light-sensitive retinal cells that are distinct from rods and cones and from signals to the SCN via the retinohypothalamic tract. Under constant conditions of light or dark, however, the SCN clock becomes "free running" and slightly drifts away from a 24-hour cycle each day.
|
| The pineal gland forms a neuroendocrine link between the SCN and various physiological processes that require circadian control. This tiny gland, close to the hypothalamus, synthesizes the hormone melatonin from the neurotransmitter serotonin, of which tryptophan is the precursor. The rate-limiting enzyme for melatonin synthesis is N-acetyltransferase. The amount and activity of this enzyme in the pineal gland vary markedly in a cyclic fashion, which accounts for the cycling of melatonin secretion and its plasma levels. Synthesis of melatonin is inhibited by light and markedly stimulated by darkness (Fig. 37-4). Thus, melatonin may transmit the information that nighttime has arrived, and body functions are regulated accordingly. Melatonin feedback to the SCN at dawn or dusk may also help evoke day-night entrainment of the SCN 24- to 25-hour clock. Melatonin has numerous other actions, including induction of sleep.
|
| Another important input to hypothalamic neurons and the pituitary gland is stress, either as systemic stress (e.g., hemorrhage, inflammation) or as processive stress (e.g., fear, anxiety). Major medical or surgical stress overrides the circadian clock and causes a pattern of persistent and exaggerated hormone release and metabolism that mobilizes endogenous fuels, such as glucose and free fatty acids, and augments their delivery to critical organs. By contrast, growth and reproductive processes are suppressed. Additionally, cytokines released during inflammatory or immune responses, or both, directly regulate the release of hypothalamic releasing hormones and pituitary hormones.
|
| page 655 |  | | page 656 |
| Figure 37-3 A circadian pacemaker directs numerous endocrine and body functions, each with its own daily profile. The nighttime rise in plasma melatonin may mediate certain other circadian patterns. (Data from Schwartz WJ: Adv Intern Med 38:81, 1994.) |
| CHEMICAL NATURE OF HORMONES
|
| Figure 37-4 Origin of circadian rhythms in endocrine gland secretion, metabolic processes, and behavioral activity. (Modified from Turek FW: Recent Prog Horm Res 49:43, 1994.) |
| Hormones are classified biochemically as proteins/peptides, catecholamines, iodothyronines, or steroid hormones. The chemical nature of a hormone determines (1) how it is synthesized, stored, and released; (2) how it is transported in blood; (3) its biological
half-life and mode of clearance; and (4) its cellular mechanism of action.
|
| Protein and peptide hormones can be grouped into structurally related molecules that are encoded by gene families. Protein/peptide hormones gain their specificity from their primary amino acid sequence and from posttranslational modifications, especially glycosylation.
|
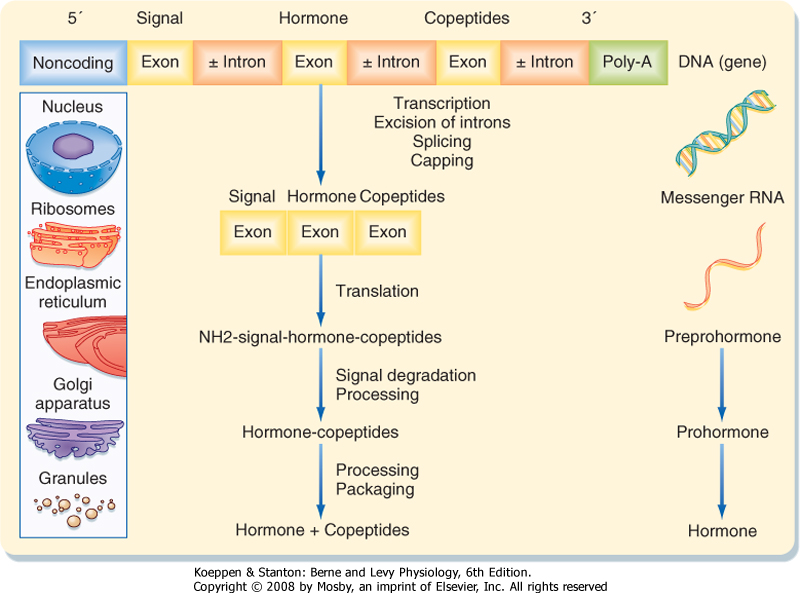
| Because protein/peptide hormones are destined for secretion outside the cell, they are synthesized and processed differently from proteins destined to remain within the cell or to be continuously added to the membrane (Fig. 37-5). These hormones are synthesized on the polyribosome as larger preprohormones or prehormones. The nascent peptides have at their N-terminal a group of 15 to 30 amino acids called the signal peptide. The signal peptide interacts with a ribonucleoprotein particle, which ultimately directs the growing peptide chain through a pore in the membrane of the endoplasmic reticulum located on the cisternal (i.e., inner) surface of the endoplasmic reticular membrane. Removal of the signal peptide by a signal peptidase generates a hormone or prohormone, which is then transported from the cisternae of the endoplasmic reticulum to the Golgi apparatus, where it is packaged into a membrane-bound secretory vesicle that is subsequently released into the cytoplasm. The carbohydrate moiety of glycoproteins is added in the Golgi apparatus.
|
| page 656 |  | | page 657 |
| Figure 37-5 Schematic representation of peptide hormone synthesis. In the nucleus the primary gene transcript, a premessenger RNA molecule, undergoes excision of introns, splicing of exons, capping of the 5' end, and addition of poly(A) at the 3' end. The resultant mature messenger RNA enters the cytoplasm, where it directs the synthesis of a preprohormone peptide sequence on ribosomes. In this process the N-terminal signal is removed, and the resultant prohormone is transferred vectorially into the endoplasmic reticulum. The prohormone undergoes further processing and packaging in the Golgi apparatus. After final cleavage of the prohormone within the granules, they contain the hormone and copeptides ready for secretion by exocytosis. |
| Most hormones are produced as prohormones. Prohormones harbor the peptide sequence of the
active hormone within their primary sequence. However, prohormones are inactive or less active and require the action of endopeptidases to trim away the neighboring inactive sequences.
|
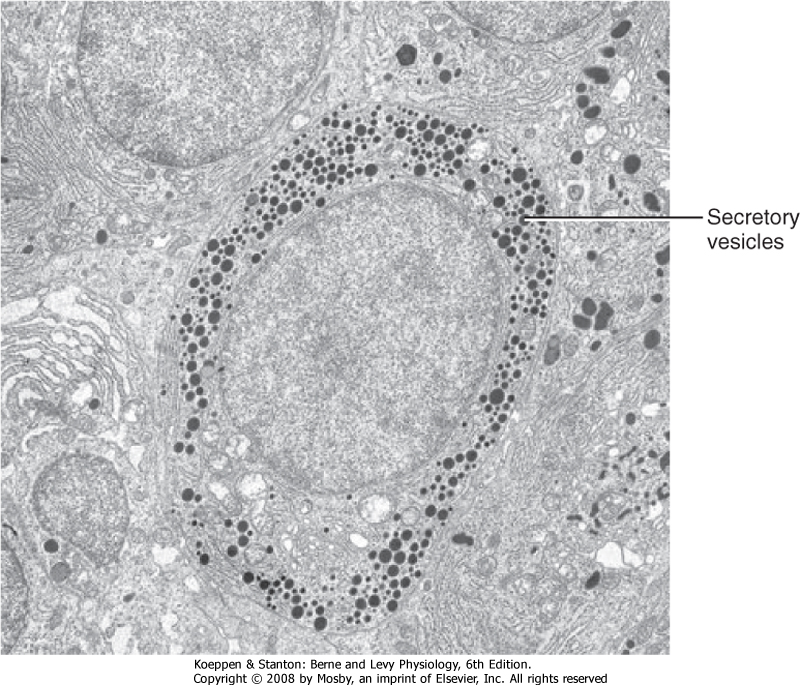
| Protein/peptide hormones are stored in the gland as membrane-bound secretory vesicles and are released by exocytosis through the regulated secretory pathway. Thus, hormones are not continually secreted. Rather, they are secreted in response to a stimulus through a mechanism of stimulus-secretion coupling. Regulated exocytosis requires energy, Ca++, an intact cytoskeleton (microtubules, microfilaments), and the presence of coat proteins that specifically deliver secretory vesicles to the cell membrane. The ultrastructure of protein hormone-producing cells is characterized by abundant rough endoplasmic reticulum and Golgi membranes and the presence of secretory vesicles (Fig. 37-6).
|
| Protein/peptide hormones are soluble in body fluids and, with the notable exceptions of IGFs and growth hormone (GH), circulate in blood predominantly in an unbound form and therefore have short biological half-lives. Protein hormones are removed from blood primarily by endocytosis and lysosomal degradation of hormone-receptor complexes (see later). Many protein hormones are small enough to appear in urine in a physiologically active form. For example, follicle-stimulating hormone (FSH) and luteinizing hormone (LH) are present in urine.
|
| Proteins/peptides are readily digested in the gastrointestinal tract if administered orally. Hence, they must be administered by injection or, in the case of small peptides, through a mucous membrane (sublingually or intranasally). Because proteins/peptides do not cross cell membranes readily, they signal through membrane receptors (see Chapter 3).
|
| page 657 |  | | page 658 |
| Bioactive hormones are generated from prohormones through proteolytic cleavage of the prohormone by prohormone (also called proprotein) convertases. The proprotein convertase family includes hfurin, hPC1, hPC2, hPACE4, and hPLC. These enzymes are expressed in a cell-specific manner. For example, insulin-producing cells (beta cells) of the pancreatic islets express both PC1 and PC2. Insulin is produced as preproinsulin, cleaved to proinsulin in the endoplasmic reticulum, and packaged in secretory vesicles as proinsulin. While in the secretory vesicle, a portion of the center of the single chain (connecting [C] peptide) is cleaved sequentially by PC1 and PC2. The mature secretory vesicle contains and secretes equimolar amounts of insulin and C peptide. Sometimes prohormones contain the sequence of multiple hormones. For example, the protein pro-opiomelanocortin (POMC) contains the amino acid sequences of adrenocorticotropic hormone (ACTH) and melanocyte-stimulating hormones (MSHs). Pituitary cells express only PC1 and release only ACTH as a bioactive peptide. In contrast, certain neuronal cell types and keratinocytes express both PC1 and PC2 and can produce MSHs. There are also prohormones, called polyproteins, that contain multiple copies of the same bioactive peptide. For example, the sequence for thyrotropin-releasing hormone (TRH) is reiterated six times within the prepro-TRH sequence. Rare mutations in PC1 have been identified in humans and are associated with extreme childhood obesity, defects in glucose homeostasis, low glucocorticoid levels, loss of menstrual cycles and hypogonadism, and problems in gastrointestinal function. |
 |
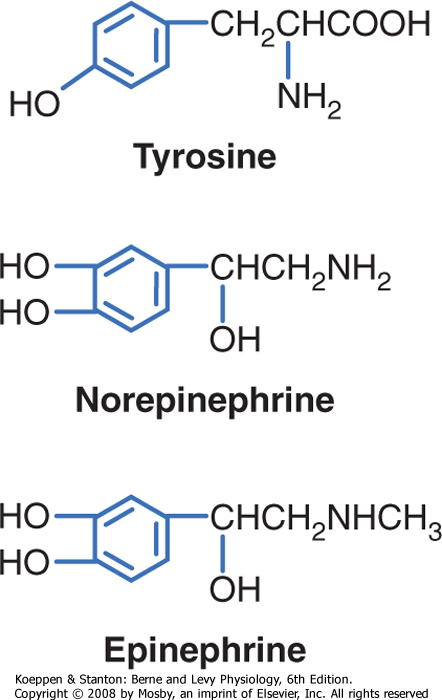
| Catecholamines are synthesized by the adrenal medulla and neurons and include norepinephrine, epinephrine, and dopamine (Fig. 37-7). The primary hormonal product of the adrenal medulla is epinephrine and, to a lesser extent, norepinephrine. Catecholamines gain their specificity through enzymatic modifications of the amino acid tyrosine. Catecholamines are stored in secretory vesicles that are part of the regulated secretory pathway. They are copackaged with ATP, Ca++, and proteins called chromogranins. Chromogranins play a role in the biogenesis of secretory vesicles and in the organization of components within the vesicles. Catecholamines are soluble in blood and circulate either unbound or loosely bound to albumin. They are similar to protein/peptide hormones in that they do not cross cell membranes readily
and hence produce their actions though membrane receptors. Catecholamines have short biological half-lives (1 to 2 minutes) and are primarily removed from blood by cell uptake and enzymatic modification.
|
| Steroid hormones are made by the adrenal cortex, ovaries, testes, and placenta. Steroid hormones from these glands fall into five categories: progestins, mineralocorticoids, glucocorticoids, androgens, and estrogens. Progestins and the corticoids are 21-carbon steroids, whereas androgens are 19-carbon steroids and estrogens are 18-carbon steroids (Table 37-2). Steroid hormones also include the active metabolite of vitamin D (see Chapter 39), which is a secosteroid (i.e., one of the rings has an open conformation).
|
| Gonadotropins refer to the pituitary hormones LH and FSH. These hormones are heterodimers that consist of a common α subunit and a unique β subunit (see Chapter 40). The urine of postmenopausal women is an excellent source of gonadotropins because postmenopausal serum gonadotropin levels are high as a result of the loss of negative feedback by ovarian steroids (see Chapter 43), and the hormones are filtered and excreted as intact molecules in urine. A third gonadotropin is the placental hormone human chorionic gonadotropin (hCG; see Chapter 43). hCG has the same common α subunit and an hCG-specific β subunit. hCG is an extremely stable hormone, and blood hCG levels double every 2 days during the first trimester. Accordingly, urinary levels of hCG also increase rapidly. Pregnancy tests are based on immunological detection in urine of the hCG-specific β subunit as part of the intact hCG heterodimer. |
 |
|
| Figure 37-6 Ultrastructure of a protein hormone-producing cell. Note the presence of secretory vesicles and rough endoplasmic reticulum in the protein hormone-secreting cell. (From Kierszenbaum AL: Histology and Cell Biology: An Introduction to Pathology, 2nd ed. Philadelphia, Mosby, 2007.) |
| page 658 |  | | page 659 |
|
Table 37-2.
Steroid Hormones |
| Family | Number of Carbons | Specific Hormone | Primary Site of Synthesis | Primary Receptor |
| Progestin | 21 | Progesterone | Ovary
Placenta | Progesterone receptor (PR) |
| Glucocorticoid | 21 | Cortisol
Corticosterone | Adrenal cortex | Glucocorticoid receptor (GR) |
| Mineralocorticoid | 21 | Aldosterone
11-Deoxycorticosterone | Adrenal cortex | Mineralocorticoid receptor (MR) |
| Androgen | 19 | Testosterone
Dihydrotestosterone | Testis | Androgen receptor (AR) |
| Estrogen | 18 | Estradiol-17β
Estriol | Ovary
Placenta | Estrogen receptor (ER) |
| Figure 37-7 Structure of catecholamines. |
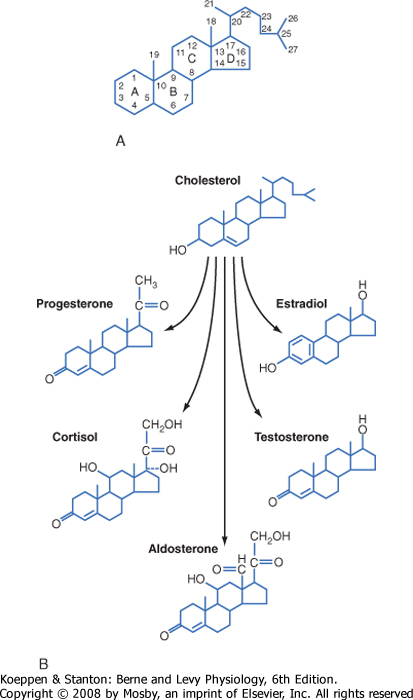
| Steroid hormones are synthesized by a series of enzymatic modifications of cholesterol and have a cyclopentanoperhydrophenanthrene ring (or a derivative thereof) as their core (Fig. 37-8). The enzymatic modifications of cholesterol are of three general types: hydroxylation, dehydrogenation/reduction, and lyase reactions. The purpose of these modifications is to produce a cholesterol derivative that is sufficiently unique to be recognized by a specific receptor. Thus, progestins bind to the progesterone receptor (PR), mineralocorticoids bind to the mineralocorticoid receptor (MR), glucocorticoids bind to the glucocorticoid receptor (GR), androgens bind to the androgen receptor (AR), estrogens bind to the estrogen receptor (ER), and the active vitamin D metabolite binds to the vitamin D receptor (VDR). The complexity of steroid hormone action is increased by the expression of multiple forms of each receptor. Additionally, there is some degree of nonspecificity between steroid hormones and the receptors that they bind to. For example, glucocorticoids bind to the MR with high affinity, and progestins, glucocorticoids, and androgens can all interact with the PR, GR, and AR to some degree. As discussed later, steroid hormones are hydrophobic and pass through cell membranes easily. Accordingly, classic steroid hormone receptors are localized intracellularly and act by regulating gene expression. Evidence is mounting for the presence of plasma membrane and juxtamembrane steroid hormone receptors that mediate rapid, nongenomic actions of steroid hormones.
|
|
| Figure 37-8 A, Structure of cholesterol, the precursor of steroid hormones. B, Structure of steroid hormones. |
| page 659 |  | | page 660 |
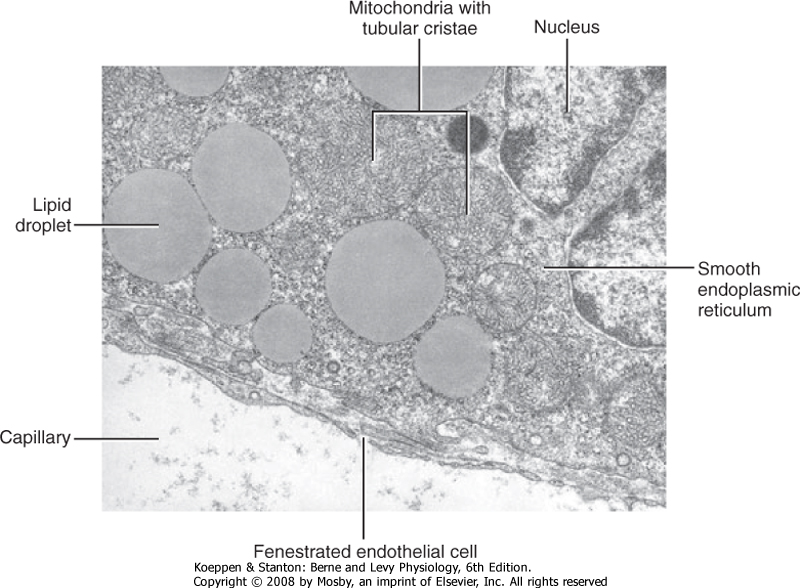
| Steroidogenic cell types are defined as cells that can convert cholesterol to pregnenolone, which is the first reaction common to all steroidogenic pathways. Steroidogenic cells have some capacity for cholesterol synthesis but often obtain cholesterol from cholesterol-rich lipoproteins (low-density lipoproteins and high-density lipoproteins). Pregnenolone is then further modified by several enzymatic reactions. Because of their hydrophobic nature, steroid hormones and precursors can leave the steroidogenic cell easily and thus are not stored. Therefore, steroidogenesis is regulated at the level of uptake, storage, and mobilization of cholesterol and at the level of steroidogenic enzyme gene expression and activity. Steroids are not regulated at the level of secretion of the preformed hormone. A clinical implication of this mode of secretion is that high levels of steroid hormone precursors are easily released into blood when a steroidogenic enzyme within a given pathway is inactive or absent. The ultrastructure of steroidogenic cells is distinct from protein- and catecholamine-secreting cells. Steroidogenic enzymes reside within the inner mitochondrial membrane or the membrane of the smooth endoplasmic reticulum. Thus, steroidogenic cells typically contain extensive mitochondria and smooth endoplasmic reticulum (Fig. 37-9). These cells also contain lipid droplets, which represent a store of cholesterol esters.
|
|
| Figure 37-9 Ultrastructure of a steroidogenic cell. Note the abundance of lipid droplets, smooth endoplasmic reticulum, and mitochondria with tubular cristae. (From Kierszenbaum AL: Histology and Cell Biology: An Introduction to Pathology, 2nd ed. Philadelphia, Mosby, 2007.) |
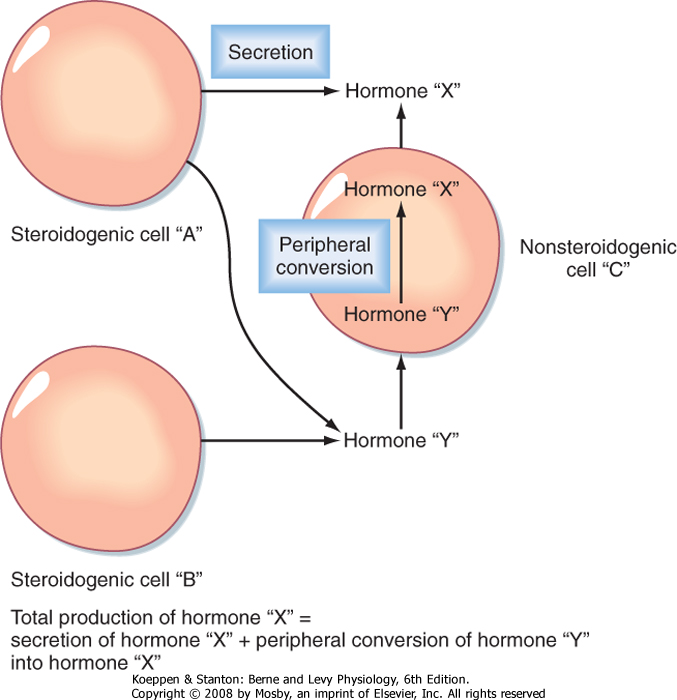
| An important feature of steroidogenesis is that steroid hormones often undergo further modifications (apart from those involved in deactivation and excretion) after their release from the original steroidogenic cell. For example, estrogen synthesis by the ovary and placenta requires at least two cell types to complete the conversion of cholesterol to estrogen. This means that one cell secretes a precursor and a second cell
converts the precursor to estrogen. There is also considerable peripheral conversion of active steroid hormones. For example, the testis secretes little estrogen. However, adipose, muscle, and other tissues express the enzyme for converting testosterone (a potent androgen) to estradiol-17β (a potent estrogen). Thus, the overall production of "steroid hormone X" is equivalent to the sum of the secretion of "steroid hormone X" from a steroidogenic cell type and peripheral conversion of other steroids to "steroid hormone X" (Fig. 37-10). Peripheral conversion can produce (1) a more active, but similar class of hormone (e.g., conversion of 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D); (2) a less active hormone that can be reversibly activated by another tissue (e.g., conversion of cortisol to cortisone in the kidney, followed by conversion of cortisone to cortisol in abdominal adipose tissue); or (3) a different class of hormone (e.g., conversion of testosterone to estrogen). Peripheral conversion of steroids plays an important role in several endocrine disorders (see Chapters 42 and 43).
|
| Because of their nonpolar nature, steroid hormones are not readily soluble in blood. Therefore, steroid hormones circulate bound to transport proteins, including albumin, but also the specific transport proteins sex hormone-binding globulin (SHBG) and corticosteroid-binding globulin (CBG) (see later). Excretion of hormones from the body typically involves inactivating modifications followed by glucuronide or sulfate conjugation in the liver. These modifications increase the water solubility of the steroid and decrease its affinity for transport proteins, thereby allowing the inactivated steroid hormone to be excreted by the kidney. Steroid compounds are absorbed fairly readily in the gastrointestinal tract and may therefore be administered orally.
|
| page 660 |  | | page 661 |
| Figure 37-10 Peripheral conversion of steroid hormones. |
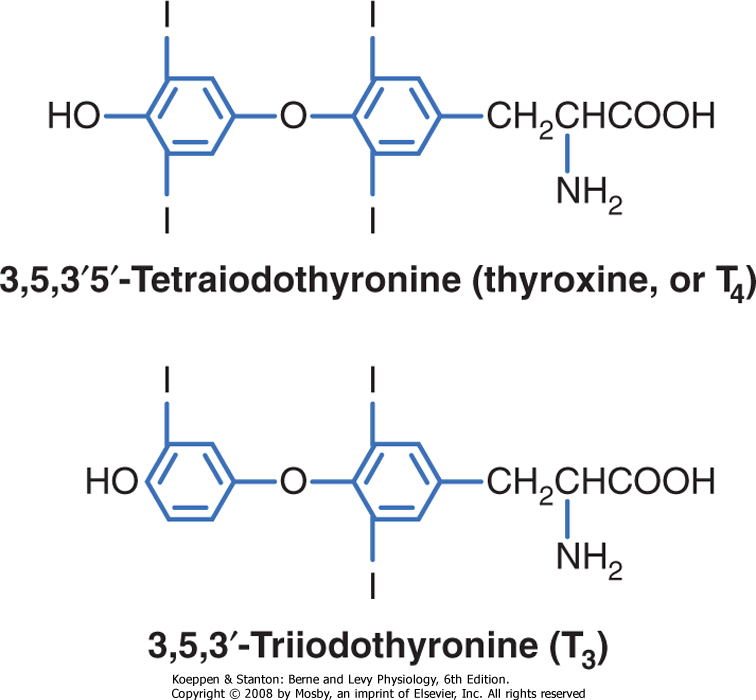
| Thyroid hormones are iodothyronines (Fig. 37-11) that are made by the coupling of iodinated tyrosine residues through an ether linkage. Their specificity is determined by the thyronine structure, as well as by where the thyronine is iodinated. Thyroid hormones cross cell membranes by both diffusion and transport systems. They are stored extracellularly in the thyroid as an integral part of the glycoprotein molecule thyroglobulin. Thyroid hormones are sparingly soluble in blood and aqueous fluids and are transported in blood bound (>99%) to serum-binding proteins. A major transport protein is thyroid hormone-binding globulin (TBG). Thyroid hormones have long half-lives (t½; thyroxine [T4] = 7 days; triiodothyronine [T3] = 18 hours). Thyroid hormones are similar to steroid hormones in that the thyroid hormone receptor (TR) is intracellular and acts as a transcription factor. In fact, the TR belongs to the same gene family that includes steroid hormone receptors and VDR. Thyroid hormones can be administered orally, and sufficient hormone is absorbed intact to make this an effective mode of therapy.
|
| TRANSPORT OF HORMONES IN THE CIRCULATION
|
| Figure 37-11 Structure of thyroid hormones, which are iodinated thyronines. |
A significant fraction of steroid and thyroid hormones is transported in blood bound to plasma proteins that are produced in a regulated manner by the liver. Protein and polypeptide hormones are generally transported free in blood. The concentrations of bound hormone (HP), free hormone (H), and plasma transport protein (P) are in equilibrium. If free hormone
levels drop, hormone will be released from the transport proteins. This relationship may be expressed as
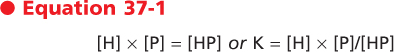
where K is the dissociation constant.
|
| Free hormone is the biologically active form for action on the target organ, feedback control, and clearance by cellular uptake and metabolism. Consequently, when evaluating hormonal status, one must sometimes determine free hormone levels rather than just total hormone levels. This is particularly important because hormone transport proteins themselves are regulated by altered endocrine and disease states.
|
| page 661 |  | | page 662 |
| Protein binding serves several purposes. It prolongs the circulating t½ of the hormone. Many hormones
cross cell membranes readily and would either enter cells or be excreted by the kidney were they not protein bound. The bound hormone represents a "reservoir" of hormone and as such can serve to "buffer" acute changes in hormone secretion. Some hormones, such as steroids, are sparingly soluble in blood, and protein binding facilitates their transport.
|
| CELLULAR RESPONSES TO HORMONES
|
| Hormones are also referred to as ligands, in the context of ligand-receptor binding, and as agonists, in that their binding to the receptor is transduced into a cellular response. Receptor antagonists typically bind to a receptor and lock it in an inactive state, unable to induce a cellular response. Loss or inactivation of a receptor results in hormonal resistance. Constitutive activation of a receptor leads to unregulated, hormone-independent activation of cellular processes.
|
| Hormones regulate essentially every major aspect of cellular function in every organ system. Hormones control the growth of cells, ultimately determining their size and competency for cell division. Hormones regulate the differentiation of cells and their ability to survive or undergo programmed cell death. They influence cellular metabolism, the ionic composition of body fluids, and cell membrane potential. Hormones orchestrate several complex cytoskeletal-associated events, including cell shape, migration, division, exocytosis, recycling/endocytosis, and cell-cell and cell-matrix adhesion. Hormones regulate the expression and function of cytosolic and membrane proteins, and a specific hormone may determine the level of its own receptor or the receptors for other hormones.
|
| Although hormones can exert coordinated, pleiotropic control on multiple aspects of cell function, any given hormone does not regulate every function in every cell type. Rather, a single hormone controls a subset of cellular functions in only the cell types that express receptors for that hormone. Thus, selective receptor expression determines which cells will respond to a given hormone. Moreover, the differentiated state of a cell will determine how it will respond to a hormone. Thus, the specificity of hormonal responses resides in the structure of the hormone itself, the receptor for the hormone, and the cell type in which the receptor is expressed. Serum hormone concentrations are typically extremely low (10-11 to 10-9 M). Therefore, a receptor must have high affinity, as well as specificity, for its cognate hormone.
|
How does hormone-receptor binding get transduced into a cellular response? Hormone binding to a receptor induces conformational changes in the receptor. This is referred to as a signal. The signal is transduced into the activation of one or more intracellular messengers. Messenger molecules then bind to effector proteins, which in turn modify specific cellular functions. The combination of hormone-receptor binding (signal), activation of messengers (transduction), and regulation of one or more effector proteins is referred to as a signal transduction pathway (also called simply a signaling pathway), and the final outcome is referred to as the cellular response. Signaling pathways are usually characterized by the following:
- Multiple, hierarchical steps in which "downstream" effector proteins are dependent on and driven by "upstream" receptors, transducers, and effector proteins. This means that loss or inactivation of one or more components within the pathway leads to general resistance to the hormone, whereas constitutive activation or overexpression of components can drive a pathway in an unregulated manner.
- Amplification of the initial hormone-receptor binding. Amplification can be so great that maximal response to a hormone is achieved on binding of hormone to a small percentage of receptors.
- Activation of multiple pathways, or at least regulation of multiple cell functions, from one hormone-receptor binding event. For example, binding of insulin to its receptor activates three separate signaling pathways. Even in fairly simple pathways (e.g., glucagon activation of adenylyl cyclase), divergent downstream events allow the regulation of multiple functions (e.g., posttranslational activation of glycogen phosphorylase and increased phosphoenolpyruvate carboxykinase [PEPCK] gene transcription).
- Antagonism by constitutive and regulated negative-feedback reactions. This means that a signal is dampened or terminated (or both) by opposing reactions and that loss or gain of function of opposing components can cause hormone-independent activation of a specific pathway, or hormone resistance.
|
| As discussed in Chapter 3, hormones signal to cells through membrane or intracellular receptors. Membrane receptors have rapid effects on cellular processes (e.g., enzyme activity, cytoskeletal arrangement) that are independent of the synthesis of new protein. Membrane receptors can also rapidly regulate gene expression through either mobile kinases (e.g., PKA, MAPKs) or mobile transcription factors (e.g., STATs, Smads). Steroid hormones have slower, longer-term effects that involve chromatin remodeling and changes in gene expression. Increasing evidence points to rapid, nongenomic effects of steroid hormones as well, but these pathways are still being elucidated.
|
| The presence of a functional receptor is an absolute requirement for hormone action, and loss of a receptor produces essentially the same symptoms as loss of hormone. In addition to the receptor, there are fairly complex pathways involving numerous intracellular messengers and effector proteins. Accordingly, endocrine diseases can arise from abnormal expression or activity, or both, of any of these signal transduction pathway components. Finally, hormonal signals can be terminated in several ways, including hormone/receptor internalization, phosphorylation/dephosphorylation, proteosomal destruction of receptor, and generation of feedback inhibitors.
|
| page 662 |  | | page 663 |
| Endocrine diseases can be broadly categorized as hyperfunction or hypofunction of a specific hormonal pathway. Hypofunction can be caused by lack of active hormone or by hormone resistance as a result of inactivation of hormone receptors or postreceptor defects. Testicular feminization syndrome is a dramatic form of hormone resistance in which the androgen receptor is mutated and cannot be activated by androgens. In patients in whom the diagnosis is not made before puberty, the testis becomes hyperstimulated because of abrogation of the negative feedback between the testis and the pituitary gland. The increased androgen levels have no direct biological effect as a result of the receptor defect. However, the androgens are peripherally converted to estrogens. Thus, individuals who are genetically male (i.e., 46,XY) have a strongly feminized external phenotype, a female sexual identity, and usually a sexual preference for males (i.e., heterosexual relative to sexual identity). Treatment involves removal of the hyperstimulated testes (which reside in the abdomen and pose a risk for cancer), estrogen replacement therapy, and counseling for the patient and, if one exists, the partner/spouse to address infertility and social/psychological distress. |
 |
- Endocrine signaling involves (1) regulated secretion of an extracellular signaling molecule, called a hormone, into the extracellular fluid; (2) diffusion of the hormone into the vasculature and circulation throughout the body; and (3) diffusion of the hormone out of the vascular compartment into the extracellular space and binding to a specific receptor within cells of a target organ.
- The endocrine system is composed of the endocrine pancreas, the parathyroid glands, the pituitary gland, the thyroid gland, the adrenal glands, and the gonads (testes or ovaries).
- Negative feedback represents an important control mechanism that confers stability on endocrine systems. Hormonal rhythms are imposed on negative-feedback loops.
- Protein/peptide hormones are produced on ribosomes and stored in endocrine cells in membrane-bound secretory granules. They typically do not cross cell membranes readily and act through membrane-associated receptors.
- Catecholamines are synthesized in the cytosol and secretory granules and do not readily cross cell membranes. They act through cell membrane - associated receptors.
- Steroid hormones are not stored in tissues and generally cross cell membranes relatively readily. They act through intracellular receptors.
- Thyroid hormones are synthesized in follicular cells and stored in follicular colloid as thyroglobulin. They cross cell membranes and associate with nuclear receptors.
- Some hormones act through membrane receptors, with their responses being mediated by G protein-associated systems (adenylyl cyclase and phosphatidylinositol), calcium-calmodulin, tyrosine kinase-containing receptor, tyrosine kinase-associated systems, or serine/threonine kinase receptor.
- Other hormones bind to nuclear receptors and act by directly regulating gene transcription.
|
 |
|